1071
Hepatic and renal ketogenesis of hyperpolarized (1-13C)butyrate: functional evidence of metabolic zonation1LIFMET, EPFL, Lausanne, Switzerland, 2Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital, Friedrich Schiller University Jena, Jena, Germany, 3Heart Center, Department of Internal Medicine II, University Hospital Bonn, Bonn, Germany
Synopsis
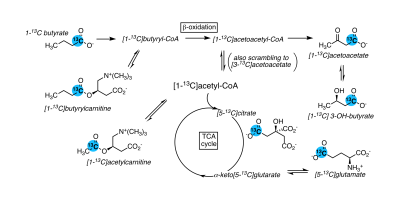
Ketones bodies are an important metabolic fuel, and butyrate is a ketogenic short-chain fatty acid. We report the metabolism of hyperpolarized (1-13C)butyrate in the rat kidney and liver. The main metabolites in the kidney include [1-13C]acetoacetate, [1-13C]butyrylcarnitine and [5-13C]glutamate, and [1-13C]acetylcarnitine, 3-hydroxy[1-13C]butyrate, [5-13C]citrate and [3-13C]acetoacetate are also detectable. Fasting results in significantly lower butyrylcarnitine/[1-13C]acetoacetate and glutamate/[1-13C]acetoacetate ratios. Ketogenesis is observed in the liver and the [1-13C]butyrylcarnitine + [1-13C]acetoacetate signal normalized to butyrate is higher with fasting. The unexpectedly low 3-hydroxy[1-13C]butyrate signals are functional evidence of metabolic zonation, with acetoacetate production and reduction occurring in different cells.
Introduction
Ketone bodies are an important metabolic fuel, particularly during fasting and starvation and are mainly produced in the liver. The kidney can use both fatty acids and ketones as fuel. 13C-labeled short-chain fatty acids are amenable to hyperpolarization for metabolic studies using dissolution dynamic nuclear polarization. The conversion of hyperpolarized [1-13C]acetate to acetylcarnitine is noted in the kidney [1] and heart [2], and hyperpolarized [1-13C]butyrate has been used to probe fatty-acid oxidation in the heart [3-5]. Butyrate is a ketogenic fatty acid, and given their important roles in ketone body metabolism, this study aimed to determine whether hyperpolarized [1-13C]butyrate can probe these pathways in the kidney and liver.Methods
Polarization – (1-13C)Butyric acid was formulated with DMSO and Finland trityl radical, and 25 µl was frozen in a PEEK sample cup with liquid nitrogen, along with 17.9 µl of 10 M NaOH for neutralization. The sample was microwave irradiated ~100 min (196.70 GHz, 60 mW) in a custom-built 7T dDNP polarizer and then dissolved with 5.5 ml of hot tris buffer in D2O with rapid automated transfer to a phase separator / infusion pump in the scanner magnet.Animals – In vivo experiments were performed with male Wistar rats (261 ± 19 g; n = 6), either in the fed state or overnight fasted. Animals were anaesthetized with isoflurane and catheters were installed in the femoral arteries and a femoral vein. Arterial blood samples were analyzed with a portable epoc system and 3-hydroxybutyrate was determined with a NovaVet meter. Breathing was measured with a pneumatic pillow, body temperature with a rectal probe, and heart rate by invasive blood pressure. Heating was provided by a hydronic system with silicone tubing placed next to the animal.
Data acquisition – Animals were scanned in a 9.4T horizontal-bore scanner equipped with a VNMRS console. Following placement in the holder, a surface coil (one-loop 1H, two-loop 13C in quadrature) was placed over the left kidney and its position verified by 1H MRI. Immediately following the IV bolus of hyperpolarized butyrate (1.4 ml), a series of pulse-acquire 13C spectra were acquired (30º BIR4 pulse, 20161.3 Hz spectral width, WALTZ-16 1H-decoupling and ~3 s repetition time, respiration gated & pulse triggered). The liver was subsequently scanned after polarizing another sample.
Data analysis – Spectra in each series containing hyperpolarized signals were summed and peaks were fit using Bayes (Washington University, St. Louis). Statistical significance was assessed using Student’s t-test. Figures were prepared in R.
Results
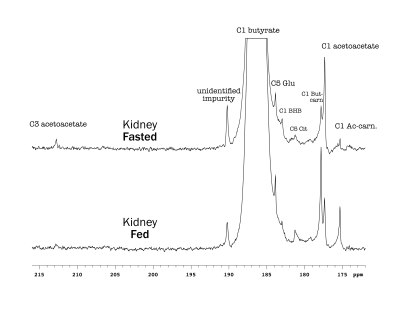
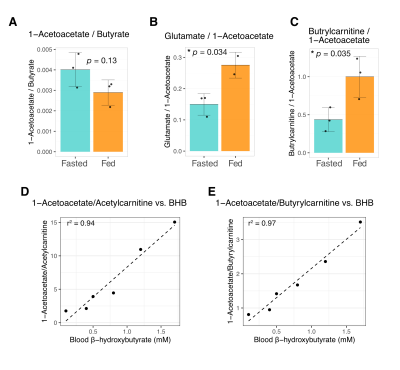
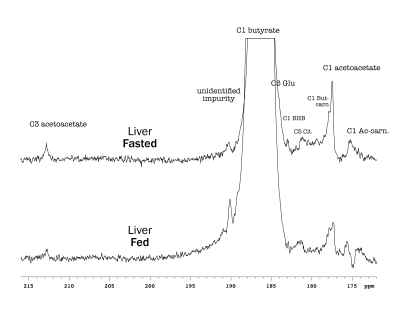
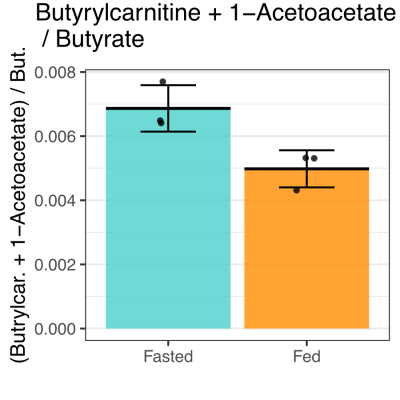
Butyrate 13C polarization was measured at 29% after dissolution and transfer to the scanner magnet. Several butyrate metabolites were detected in the kidney, most prominently [1-13C]acetoacetate, [1-13C]butyrylcarnitine and [5-13C]glutamate, but also [1-13C]acetylcarnitine, 3-hydroxy[1-13C]butyrate, [5-13C]citrate and [3-13C]acetoacetate (Figure 1). The [1-13C]acetoacetate : [1-13C]butyrate ratio trended higher with fasting (Figure 2A) and significant differences were apparent in the metabolite ratios. The glutamate : [1-13C]acetoacetate ratio was 1.8-fold higher in the fed state (p = 0.034) (Figure 2B), and the butyrylcarnitine : [1-13C]acetoacetate ratio was similarly 2.3-fold higher (p = 0.035) (Figure 2C). Additionally, the [1-13C]acetoacetate : acetylcarnitine (Figure 2D) and [1-13C]acetoacetate : butyrylcarnitine (Figure 2E) ratios correlated well with blood 3-hydroxybutyrate levels.Spectral resolution was lower in the liver, but the main metabolites seen in the kidney are also apparent (Figure 3). Any [5-13C]glutamate signal was generally obscured by the broader [1-13C]butyrate peak. Small [3-13C]acetoacetate signals were observed, as was 3-hydroxy[1-13C]butyrate. The neighboring butyrylcarnitine and [1-13C]acetoacetate peaks were not well resolved and were fit as a single peak. Normalized to butyrate, the butyrylcarnitine and [1-13C]acetoacetate signals were 1.4-fold higher in the fasted case (p = 0.024).
Discussion
Higher butyrate polarization provides increased sensitivity and ketogenesis in both the kidney and liver can be detected. The predominant labeling of acetoacetate at the 1-position versus the 3-position indicates that ketogenesis from butyrate occurs mostly directly from fatty acid oxidation. Labeling at the 3-position results from (pseudo)ketogenesis after conversion of [1-13C]butyrate to [1-13C]acetyl-CoA.Compared to the heart, where acetylcarnitine is the main butyrate metabolite, the prominence of butyrylcarnitine and [1-13C]acetoacetate in the liver and kidney is consistent with ketogenesis being the predominant pathway in these tissues. 3-Hydroxybutyrate is the main ketone body in circulation, and the 3-hydroxybutyrate : acetoacetate ratio is used as a measure of liver mitochondrial redox state. The larger acetoacetate and minor 3-hydroxy[1-13C]butyrate signals seen in the liver and kidney indicate that they do not reflect the steady-state levels of the metabolites. Non-hyperpolarized MR experiments of hepatic [1-13C]butyrate metabolism show that 3-hydroxy[1-13C]butyrate is the predominant ketone body metabolite after ~1 hr of butyrate infusion [6]. The predominant conversion to hyperpolarized [1-13C]acetoacetate in the first minute following infusion can be explained by metabolic zonation, wherein oxidation of butyrate to acetoacetate and reduction of acetoacetate to 3-hydrobutyrate take place in different cellular populations. Periportal hepatocytes carry out fatty acid oxidation and ketogenesis [7], while 3-hydroxybutyrate dehydrogenase activity is mainly in perivenous hepatocytes [8,9].
Conclusions
Hyperpolarized (1-13C)butyrate is able to probe fatty acid oxidation to ketone bodies and TCA-cycle metabolites in the kidney, and it can probe ketogenesis in the liver. The higher temporal resolution afforded by the enhanced signal provides functional evidence for metabolic zonation in ketogenesis.Acknowledgements
We gratefully acknowledge Stefan Mitrea, Analina Hausin and Mario Lepore of the Centre d’imagerie biomédicale at the EPFL for their expert assistance with the animal experiments.References
- Koellisch U, Laustsen C, Nørlinger TS, Ostergaard JA, Flyvbjerg A, Gringeri CV, Menzel MI, Schulte RF, Haase A, Stødkilde-Jørgensen H (2015) Investigation of metabolic changes in STZ-induced diabetic rats with hyperpolarized [1-13C]acetate. Physiol Rep. doi: 10.14814/phy2.12474
- Bastiaansen JAM, Cheng T, Lei H, Gruetter R, Comment A (2015) Direct noninvasive estimation of myocardial tricarboxylic acid cycle flux in vivo using hyperpolarized C-13 magnetic resonance. J Mol Cell Cardiol 87:129–137.
- Ball DR, Rowlands B, Dodd MS, Le Page L, Ball V, Carr CA, Clarke K, Tyler DJ (2014) Hyperpolarized butyrate: a metabolic probe of short chain fatty acid metabolism in the heart. Magn Reson Med 71:1663–1669.
- Bastiaansen JAM, Merritt ME, Comment A (2016) Measuring changes in substrate utilization in the myocardium in response to fasting using hyperpolarized [1-(13)C]butyrate and [1-(13)C]pyruvate. Sci Rep 6:25573.
- Abdurrachim D, Teo XQ, Woo CC, Ong SY, Salleh NF, Lalic J, Tan RS, Lee PTH (2019) Cardiac metabolic modulation upon low-carbohydrate low-protein ketogenic diet in diabetic rats studied in vivo using hyperpolarized 13 C pyruvate, butyrate and acetoacetate probes. Diabetes Obes Metab 21:949–960.
- Pahl-Wostl C, Seelig J (1986) Metabolic pathways for ketone body production. Carbon-13 NMR spectroscopy of rat liver in vivo using carbon-13-multilabeled fatty acids. Biochemistry 25:6799–6807.
- Hou Y, Hu S, Li X, He W, Wu G (2020) Amino Acid Metabolism in the Liver: Nutritional and Physiological Significance. In: Amino Acids in Nutrition and Health. Springer, Cham, pp 21–37
- Braakman I, Keij J, Hardonk MJ, Meijer DKF, Groothuis GMM (1991) Separation of periportal and perivenous rat hepatocytes by fluorescence-activated cell sorting: Confirmation with colloidal gold as an exogenous marker. Hepatology 13:73–82.
- Katz NR, Fischer W, Giffhorn S (1983) Distribution of enzymes of fatty acid and ketone body metabolism in periportal and perivenous rat-liver tissue. Eur J Biochem 135:103–107.
Figures