1069
Seiffert spirals for hyperpolarized 13C MRI with efficient k-space sampling and flexible acceleration1Department of Health Technology, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Core Facility Small Animal MRI, University of Ulm, Ulm, Germany, 3MR Research Centre, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 4Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 5Department of Internal Medicine II, University of Ulm, Ulm, Germany
Synopsis
The Seiffert spirals trajectory has favorable properties for hyperpolarized 13C MRI. It allows for high degrees of undersampling and combined conjugate gradient compressed sensing SENSE reconstruction when multi-channel arrays are used. The trajectory was evaluated in simulations, in phantom, and for in vivo hyperpolarized 13C-urea MRI of pig kidneys with retrospective undersampling and high temporal resolution. For single-frequency imaging, flexible acceleration is achieved through reconstruction based on any number and combination of spiral arms. Simulations demonstrated noise-like aliasing and successful reconstruction. The in vivo experiment showed improved characterization of urea dynamics after undersampling. The reconstruction algorithm can be further improved.
Introduction
Hyperpolarized MR acquisition requires fast and efficient sampling to maximize information output. Acceleration approaches applied include advanced k-space trajectories,1 compressed sensing,2 and parallel imaging.3,4 We propose to use Seiffert spirals in a scheme that includes all three acceleration approaches. The recently presented Seiffert spirals trajectory5 has many favorable properties for hyperpolarized imaging. It samples k-space in a 3D center-out manner with low-discrepancy coverage and few excitations per volume. Furthermore, it has an advantageous incoherent aliasing pattern when undersampled, allowing combined compressed sensing and parallel imaging reconstruction.We have designed a Seiffert spirals trajectory for metabolite-specific hyperpolarized 13C MRI and have evaluated it through simulations, a phantom experiment, and an in vivo pig kidney experiment.
Methods
The Seiffert spirals trajectory designed for this study has: FOV=26x26x26cm3, matrix size=64x64x64, spiral arms=25, readout duration=45ms, gradient amplitude maximum=41mT/m, and slew rate maximum=180T/m/s.Data were reconstructed with combined compressed sensing and SENSE using a non-linear conjugate gradient algorithm (CG-CS-SENSE) with backtracking line-search. The algorithm is based on code by Michael Lustig.6 Given k-space measurements y, and Fourier operator F, the algorithm finds the image x that minimizes:
$$Φ(x)=||\mathbf{F}·\mathbf{W}'·x-y||^2+λ_1·|x|_1+λ_2·\mathbf{TV}·(\mathbf{W}'·x).$$
W is a wavelet operator (Daubechies), TV is a total variational operator, and $$$λ_1$$$ and $$$λ_2$$$ are regularization parameters. F includes sensitivity-weighted coil combination7 and B0 off-resonance correction.8 All CG-CS-SENSE reconstructions used $$$λ_1$$$=0.1 and $$$λ_2$$$=0.05 with maximum 10 CG iterations (stopping criteria: norm of update<0.01).
For simulations and phantom acquisitions, a home-built 28-channel 13C receive array was used.9 For in vivo a home-built flexible 8-channel 13C receive array10 was used that allows 13C sensitivity mapping at the 23Na frequency.11 A 13C clamshell coil was used for transmission (RAPID). For phantom acquisitions a home-built 13C transmit coil was used.
Simulations
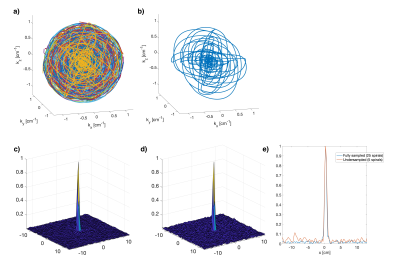
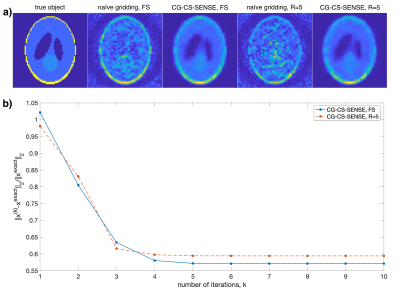
The spatial PSF was simulated numerically (MATLAB) with no B0 off-resonance and with T2*=45ms. PSFs were reconstructed for the fully sampled Seiffert spirals and with R=5 using linear 3D gridding with density compensation. Shepp Logan simulations were performed with added complex noise, the above B0 and T2*, and using simulated sensitivity profiles for the 28-channel array. B1- intensity correction was applied after reconstruction.
Phantom experiments
Both phantom and in vivo imaging were performed using a MR750 3T scanner (GE Healthcare).
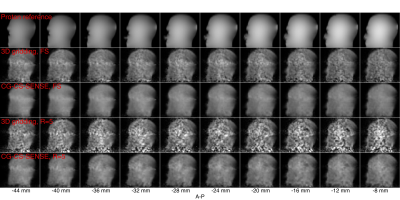
The sequence was first evaluated in a head-shaped phantom12 with FOV=26cm, slab thickness=260mm, TR=1000ms, flip angle=70° (spectral-spatial13), scan time=53.3min. Data were reconstructed using simulated sensitivity maps registered via coil markers as previously described.4 B1- intensity correction was also applied.
In vivo experiment
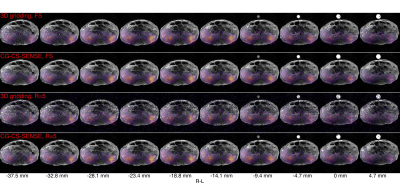
The animal experiment was approved by the Danish Animal Inspectorate and was performed on a 40kg, healthy, and anaesthetized (Propofol and Fentanyl) female Danish Landrace pig. Polarization was done as previously described14 to result in 55mL (180 mM) of hyperpolarized 13C,15N2-urea solution that was injected over 10s. Imaging was started at the end of injection.
Seiffert spirals acquisition: FOV=30cm, slab thickness=160mm, TR=60ms, (soft pulse) flip angle=8°, scan time=1min.
A 23Na 3D cones-based ultra-short echo time sequence was used for sensitivity mapping, with isotropic FOV=48cm, matrix=120x120x120, TR=120ms, non-selective excitation, and scan time 1.5min. Sensitivity maps were estimated by normalizing the 3D-gridded images with the coil-combined image.
Results
Fig. 1 shows the k-space trajectory and simulated PSF. Fig. 1f demonstrates the noise-like aliasing of the Seiffert spirals when undersampled.Shepp Logan simulation results are shown in Fig. 2 with fast convergence (Fig. 2b). Fig. 2a shows clear image quality improvement after CG-CS-SENSE reconstruction and similar results for the undersampled reconstruction.
Fig. 3 shows results from the phantom acquisition. The CG-CS-SENSE reconstruction improves the undersampled image quality, however, with five times shorter acquisition the images are still noisy. B0 distortions are present near the nose due to an air bubble and at the back of the head due to coil electronics combined with a thin phantom shell (2 mm).
Fig. 4 shows the hyperpolarized 13C-urea kidney images for a single timepoint. Solid state urea polarization was 43%. In vivo kidney signal, however, was low, possibly due to excitation of the inflowing bolus in the vein. A delayed acquisition could have preserved more signal.
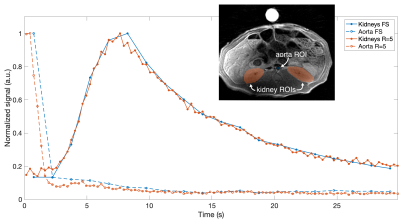
Fig. 5 shows the urea dynamics across kidney and aorta volumes-of-interest (VOIs). With the five-times higher temporal resolution of the undersampled data, a more accurate timing of the signal measured at aorta is achieved.
Discussion
A Seiffert spirals sequence was designed and applied for hyperpolarized 13C imaging. With sufficient SNR, high undersampling rates can be applied, e.g. in the post-processing for single-frequency imaging such as 13C-urea. Acceleration of R=5 was demonstrated for two different coil setups (in phantom and in vivo).Reconstruction parameters for CG-CS-SENSE reconstruction were chosen empirically with minimal optimization effort. With adapted parameters improvements are expected.
The signal measured at the aorta VOI in Fig. 5 is likely contaminated by high signal from the vein. Nevertheless, the curves still demonstrate potential benefit of higher temporal resolution.
Conclusion
Seiffert spirals was demonstrated in simulations, phantom, and in vivo for 13C imaging. For hyperpolarized 13C imaging, Seiffert spirals can be highly undersampled and reconstructed with CG-CS-SENSE when multi-channel arrays are used. This was demonstrated for 13C-urea imaging in pig kidneys with improved characterization of signal dynamics.Acknowledgements
This work has been partly funded by the Danish Council for Independent Research (DFF – 4005-00531), the Danish National Research Foundation (DNRF124) and Lundbeck Foundation (R272-2017-4023 & R278-2018-620). Volker Rasche was funded by the EU Grant Agreement: 858149.References
1. Wang J, Wright AJ, Hu D, Hesketh R, Brindle KM. Single Shot Three-Dimensional Pulse Sequence for Hyperpolarized 13C MRI. Magn. Reson. Med. 2017;77:740–752.
2. Chen H-Y, Larson PE, Gordon JW, et al. Technique development of 3D dynamic CS-EPSI for hyperpolarized 13C pyruvate MR molecular imaging of human prostate cancer. Magn. Reson. Med. 2018;80(5):2062–2072.
3. Gordon JW, Hansen RB, Shin PJ, Feng Y, Vigneron DB, Larson PE. 3D Hyperpolarized C-13 EPI with Calibrationless Parallel Imaging. J. Magn. Reson. 2018;289:92–99.
4. Olin RB, Sánchez-Heredia JD, Schulte RF, et al. Three-dimensional accelerated acquisition for hyperpolarized 13C MR with blipped stack-of-spirals and conjugate-gradient SENSE. Magn. Reson. Med. 2020;00:1–16.
5. Speidel T, Metze P, Rasche V. Efficient 3D low-discrepancy k-space sampling using highly adaptable Seiffert Spirals. IEEE Trans. Med. Imaging 2018;38(8):1833–1840.
6. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007;58(6):1182–1195.
7. Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in Sensitivity Encoding With Arbitrary k-Space Trajectories. Magn. Reson. Imaging 2001;46:638–651.
8. Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans. Med. Imaging 2003;22(2):178–188.
9. Sanchez JD, Ali A, Wang W, Olin RB, Zhurbenko V, Ardenkjær-Larsen JH. Fixed Geometry 28-Channel Array for Full Head Coverage of Hyperpolarized 13C MRS. In: Proceedings of the International Society for Magnetic Resonance in Medicine. ; 2020. p. 4106.
10. Sánchez-Heredia JD, Wang W, Grist JT, et al. Flexible 8-Channel Array for Hyperpolarized 13C at 3T (32.1 MHz), with Nearly Identical 23Na (33.8 MHz) Sensitivity Profiles. In: Proc. Intl. Soc. Mag. Reson. Med. ; 2021. p. 2509.
11. Sanchez-Heredia JD, Olin RB, Grist JT, et al. RF Coil Design for Accurate Parallel Imaging on 13C MRSI using 23Na Sensitivity Profiles (submitted for publication).; 2021.
12. Sánchez-Heredia JD, Olin RB, McLean MA, et al. Multi-Site Benchmarking of Clinical 13C RF Coils at 3 T. J. Magn. Reson. 2020;318:1–11.
13. Schulte RF, Sperl JI, Weidl E, et al. Saturation-recovery metabolic-exchange rate imaging with hyperpolarized [1-13C] pyruvate using spectral-spatial excitation. Magn. Reson. Med. 2013;69(5):1209–1216.
14. Hansen ESS, Stewart NJ, Wild JM, Stødkilde-Jørgensen H, Laustsen C. Hyperpolarized 13C,15N2-Urea MRI for assessment of the urea gradient in the porcine kidney. Magn. Reson. Med. 2016;76(6):1895–1899.
Figures