1068
Parallel imaging of hyperpolarized 13C MRI using 23Na sensitivity profiles1Department of Health Technology, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 3Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 4Department of Radiology, Oxford University Hospitals Trust, Oxford, United Kingdom, 5Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 6Department of Electrical Engineering, Technical University of Denmark, Kgs. Lyngby, Denmark, 7MR Research Centre, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 8GE Healthcare, Munich, Germany
Synopsis
Parallel imaging can aid hyperpolarized 13C MRI in extracting more information in the limited time window of the hyperpolarized signal. Using a dedicated, flexible 13C receive coil design with coupling coefficients matched for both 13C and 23Na, sensitivity profiles for reconstruction can be acquired at the 23Na frequency. We demonstrate this method in two hyperpolarized in vivo experiments involving pig kidneys and human brain using a two-times accelerated 3D blipped stack-of-spirals sequence with dual-resolution. The results show good SNR, coverage, and resolution. The method is promising for integrating and automating parallel imaging for hyperpolarized 13C MRI.
Introduction
Hyperpolarized 13C MRI needs clever acquisition strategies that take advantage of available priors to enable dynamic imaging with sufficient coverage and resolution in the short signal lifetime. Multi-channel coils can facilitate parallel imaging. However, obtaining receive coil sensitivity maps for hyperpolarized parallel imaging is challenging and yet to be established. Proposed methods include auto-calibration,1–3 self-calibration,4 and pre-calibration.5 But auto-calibration can be problematic for hyperpolarized imaging with changes in signal distribution, self-calibration depends on specialized sampling, and pre-calibration is limited to rigid coils.We instead suggest acquiring 13C coil profiles through sensitivity mapping at the 23Na frequency, which takes advantage of the close 13C (32.1 MHz at 3T) and 23Na (33.8 MHz) resonance frequencies. This allows calibration within the same scan session using the high endogenous 23Na concentration in vivo. A previous study shows that a commercial 13C clamshell transmit coil can be used for 23Na transmission,6 however, for 13C arrays a dedicated design is required to assure similar sensitivity profiles. We designed an 8-channel flexible coil to achieve that purpose. Parallel imaging was tested in phantom and for hyperpolarized [1-13C]pyruvate imaging of pig kidneys and the brain of a healthy human volunteer.
Methods
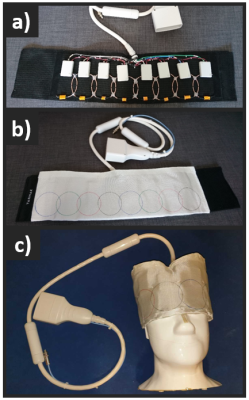
The 8-channel array (Figure 1) was designed with coil coupling coefficients matched to be nearly identical at the two frequencies. This was achieved by enforcing a high level of decoupling through mismatched preamplifiers.7 The frequency response was then adjusted between the 13C and 23Na frequencies. Each loop was built with standard flexible copper coax (RG-316), where the outer jacket created the conductive loop.All MR experiments were performed using a 3T GE MR750 MRI (GE Healthcare).
A 3D 13C blipped stack-of-spirals (R = 2) sequence8 was designed in two versions for hyperpolarized 13C MR imaging of pig kidneys (FOV=28x28x10 cm3) and human brain (FOV=28x28x12 cm3), respectively. The sequences were designed with twice the in-plane resolution for pyruvate (pig, matrix=70x70x10; human, matrix=40x40x8) compared to the metabolites.9 CG-SENSE reconstruction was done as previously described8 including B0 off-resonance correction.5,10
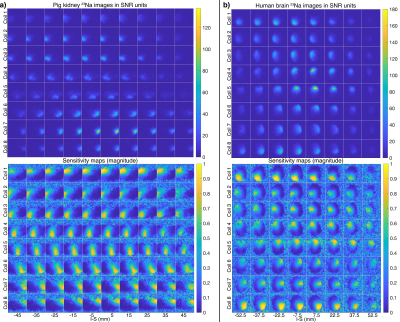
A 23Na 3D cones-based ultra-short echo time sequence was used for sensitivity mapping, with FOV=48x48x48 cm3, matrix=120x120x120, TR=120 ms, TE=0.5 ms, non-selective excitation, and scan time 1.5 min. The 23Na data were reconstructed using 3D gridding. Sensitivity maps were estimated by normalizing the multi-channel images with the root-mean-squares-combined image and performing edge-preserving smoothing.
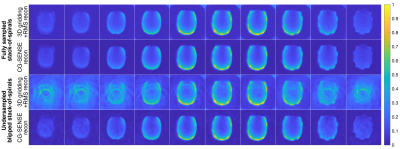
The kidney sequence was first evaluated in a head-shaped phantom11 using TR=1000 ms, flip angle=70°, and scan time=25 min. For comparison, a fully sampled stack-of-spirals adaptation was also tested.
All 13C experiments applied spectral-spatial excitation12 with TE=9 ms. For the pig experiment, 9° flip angles were used on pyruvate and 34° on metabolites. Pyruvate was acquired with a temporal resolution of 0.87 s, while the metabolites (lactate, bicarbonate, and alanine) had a temporal resolution of 2.6 s. For the human experiment, pyruvate was excited with 10° and metabolites with 28°. Pyruvate and metabolites were acquired at a temporal resolution of 1.2 s.
The animal experiment was approved by the Danish Animal Inspectorate and the human experiment by the Danish Medicines Agency and the Ethics Committee of the Central Region of Denmark. The pig experiment was performed on a 40 kg, healthy, and anaesthetized (Propofol and Fentanyl) female Danish Landrace pigs. For hyperpolarized MRI, [1-13C]pyruvic acid was polarized (SPINlab, GE Healthcare) with AH111501 (EPA). The sample was dissolved with water and buffered to physiological temperature, acidity, and osmolarity. For the human experiment, pyruvate was filtered and quality controlled. Pyruvate (250 mM) was injected at 0.43 ml/kg and chased by 20 ml saline. Imaging was started at the end of pyruvate injection.
Routine proton images, including B0 field maps, were acquired with the body coil.
Results
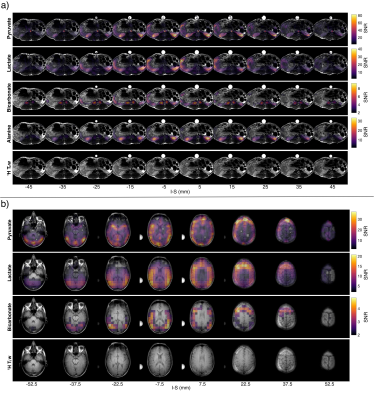
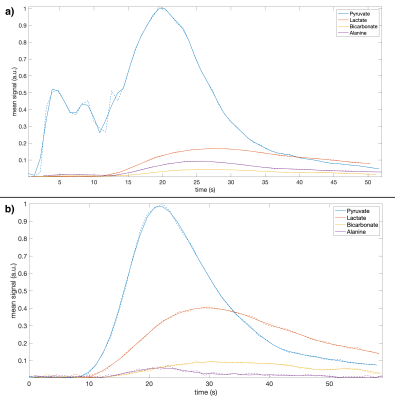
The phantom results in Figure 2 demonstrate the method, while verifying the blipped stack-of-spirals. Undersampled and fully sampled CG-SENSE reconstructions are similar with limited residual aliasing for the undersampled data.Figure 3 shows the 23Na SNR level and sensitivity maps for the in vivo acquisitions. Figure 4 shows the results of the hyperpolarized 13C in vivo experiments with metabolite images summed over time and overlayed on proton images with structural consensus and good SNR. Figure 5 shows the temporal dynamics.
Discussion
We have demonstrated parallel imaging for 13C MRI using 23Na sensitivity profiles acquired with the same coil. The coil shows good 13C SNR and sufficient 23Na SNR for fast sensitivity mapping. The flexible design allowed acquisition for both pig kidney and human brain. For the phantom, the tight fit combined with a thin shell (2 mm) resulted in “spikes” (last slices in Figure 2) caused by coil electronics distorting the B0.With the proposed method, parallel imaging of hyperpolarized 13C can be automated. It requires a specialized coil design, but with flexible fitting one coil can fit many purposes. The coil design will, however, not work equally well for any geometry and channel count.
Conclusion
By means of a dedicated coil design parallel imaging for hyperpolarized 13C MRI can be performed with sensitivity profiles acquired at the 23Na frequency in the same scan session using the same coil. This was demonstrated with R=2 accelerated acquisition in pig kidneys and human brain with good SNR, coverage, and resolution.Acknowledgements
This work has been partly funded by the Danish Council for Independent Research (DFF – 4005- 00531), the Danish National Research Foundation (DNRF124) and Lundbeck Foundation (R272-2017-4023 & R278-2018-620). Dr James Grist was funded by the Oxford BHF Centre of Research Excellence (RE/18/3/34214), and by a European Commission grant (858149, AlternativesToGd). Prof Damian Tyler was funded by a British Heart Foundation Senior Research Fellowship (FS/19/18/34252).
References
1. Arunachalam A, Whitt D, Fish K, et al. Accelerated spectroscopic imaging of hyperpolarized C-13 pyruvate using SENSE parallel imaging. NMR Biomed. 2009;22(8):867–873.
2. Ohliger MA, Larson PE, Bok RA, et al. Combined parallel and partial Fourier MR reconstruction for accelerated 8-channel hyperpolarized carbon-13 in vivo magnetic resonance Spectroscopic imaging (MRSI). J. Magn. Reson. Imaging 2013;38(3):701–713.
3. Jiang W, Lustig M, Larson PE. Concentric rings k-space trajectory for hyperpolarized 13C MR spectroscopic imaging. Magn. Reson. Med. 2016;75:19–31.
4. Gordon JW, Hansen RB, Shin PJ, Feng Y, Vigneron DB, Larson PE. 3D Hyperpolarized C-13 EPI with Calibrationless Parallel Imaging. J. Magn. Reson. 2018;289:92–99.
5. Hansen RB, Sánchez-Heredia JD, Bøgh N, et al. Coil profile estimation strategies for parallel imaging with hyperpolarized 13C MRI. Magn. Reson. Med. 2019;82(6):2104–2117.
6. Grist JT, Hansen ESS, Sánchez-Heredia JD, et al. Creating a clinical platform for carbon‐13 studies using the sodium‐23 and proton resonances. Magn. Reson. Med. 2020;(February):1–11.
7. Sánchez-Heredia JD, Johansen DH, Hansen RB, et al. Improved Decoupling for Low Frequency MRI Arrays using Non-conventional Preamplifier Impedance. IEEE Trans. Biomed. Eng. 2018.
8. Olin RB, Sánchez-Heredia JD, Schulte RF, et al. Three-dimensional accelerated acquisition for hyperpolarized 13C MR with blipped stack-of-spirals and conjugate-gradient SENSE. Magn. Reson. Med. 2020;00:1–16.
9. Gordon JW, Milshteyn E, Bok R, Vigneron DB, Larson PE. A Variable Resolution Echo-Planar Imaging Approach for Improved Quantification of Hyperpolarized 13C Metabolism. In: Experimental Nuclear Magnetic Resonance Conference. ; 2019.
10. Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans. Med. Imaging 2003;22(2):178–188.
11. Sánchez-Heredia JD, Olin RB, McLean MA, et al. Multi-Site Benchmarking of Clinical 13C RF Coils at 3 T. J. Magn. Reson. 2020;318:1–11.
12. Schulte RF, Sperl JI, Weidl E, et al. Saturation-recovery metabolic-exchange rate imaging with hyperpolarized [1-13C] pyruvate using spectral-spatial excitation. Magn. Reson. Med. 2013;69(5):1209–1216.
Figures