1067
Evidence of Lactate Shuttling in the Human Brain with Hyperpolarized 13C-MRI1Medical Biophysics, University of Toronto, Mississauga, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Radiation oncolocy, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4GE Healthcare, Toronto, ON, Canada, 5Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 6Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 7Radiology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 8Neurology and Hurvitz Brain Sciences Research Program Sciences, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Synopsis
It is well known that glucose is the primary source of energy in the brain, but mounting evidence suggests that at least some of this glucose is first converted to lactate and shuttled between cellular compartments before being oxidized in the TCA cycle. In this study, the hypothesis that this ”lactate shuttle” contributes to the 13C-lactate and 13C-bicarbonate signal observed in the awake human brain is tested using hyperpolarized 13C MRI (HP13C-MRI).
Introduction
It is well known that glucose is the primary source of energy in the brain, but mounting evidence suggests that at least some of this glucose is first converted to lactate and shuttled between cellular compartments before being converted back to pyruvate and oxidized in the TCA cycle [1, 2]. In this study, the hypothesis that this ”lactate shuttle” contributes to the 13C-lactate and 13C-bicarbonate signal observed in the awake human brain is tested using hyperpolarized 13C MRI (HP13C-MRI).HP13C-MRI is a methodology for metabolic profiling in vivo. Following the intravenous injection of 13C-pyruvate, the downstream metabolites 13C-lactate and 13C-bicarbonate can be readily imaged in the human brain [3]. Signal from 13C-lactate shows intracellular conversion of 13C-pyruvate via the enzyme lactate dehydrogenase and is higher with increased local rate of lactate production and/or increased local lactate pool size.
The 13C-bicarbonate signal is from bicarbonate formed when 13C-pyruvate is converted to acetyl-CoA on the mitochondrial membrane, with acetyl-CoA going on to be oxidized in the TCA cycle. Thus 13C-bicarbonate signal indirectly shows flux of 13C-pyruvate into mitochondrial oxidation.
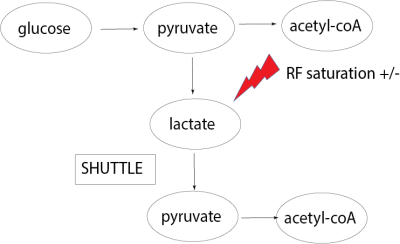
However, if lactate shuttling is occurring at a significant level in the human brain, some fraction of the 13C-bicarbonate will be formed from 13C-lactate that has been shuttled, converted back to 13C-pyruvate and consumed in the TCA cycle in a different compartment (see Figure 1). In this study, this is tested by performing two consecutive HP13C-MRI scans with two different flip angles (46o vs 80o) applied when imaging 13C-lactate. Increased 13C-bicarbonate signal with the lower flip angle on 13C-lactate (and thus less RF saturation) would be evidence consistent with lactate shuttling.
Methods
Data from six healthy volunteers (ages 23-48) were collected and analyzed for this study. All individuals were screened through the Montreal Cognitive Assessment (MoCA), and deemed cognitively normal. Informed written consent was acquired from all subjects, and the study was conducted under a protocol approved by the Sunnybrook Research Ethics Board as well as Health Canada as a Clinical Trial Application.The scan protocol was the same as used in prior human brain studies by our group, [3, 4], except with two 13C-pyruvate injections and scans for each subject, 30 minutes apart. The second set of 13C-metabolite images were acquired after anatomical image acquisition, with an altered flip angle on the just the 13C-lactate imaging interleave of the spectral-spatial 3D-EPI sequence (either 46o or 80o net flip angle per volume acquisition, with the order of the two varied).
Image reconstruction was performed in MATLAB (The MathWorks Inc., Natick, MA). To compare the regional 13C-metabolite signal between subjects, the T1-weighted images were parcellated into anatomical regions using the SLANT (Spatially Localized Atlas Network Tiles) method [5]. The parcellation produces a map of the 132 regions in the BrainCOLOR labelling protocol [6] for each subject. To normalize for the variable polarization of the 13C-pyruvate substrate at the time of delivery and during the 60 second data aquisition, each regional 13C-metabolite signal was first integrated over the 12 timepoints and divided by the time integral of the 13C-pyruvate in the same region, giving the metabolite ratios LP (the integrated 13C-lactate divided by the integrated 13C-pyruvate) and BP (the integrated 13C-bicarbonate divided by the integrated 13C-pyruvate) for each region and subject. The mean regional 13C-metabolite signals were computed with the FreeSurfer package mri_segstats (http://nmr.mgh.harvard.edu).
Results and Discussion
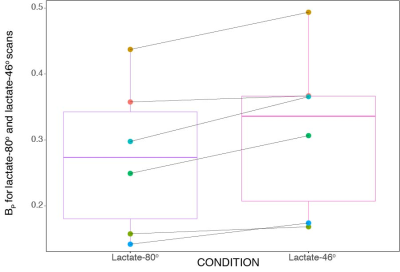
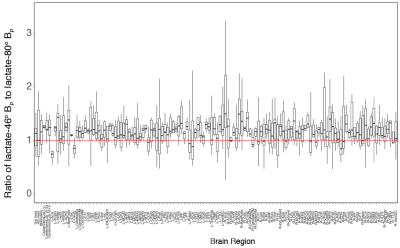
Figure 2 is a boxplot for the lactate-80o and lactate-46o conditions, where each point is the mean BP for that corresponding individual. An ANOVA was also done on the acquired data and showed that the mean BP between lactate-46o and lactate-80o scans was significantly different (p < 2e−16). No significant interaction between region and condition was observed.The ratio of lactate-46o BP to lactate-80o BP was created for each of the parcellated 132 regions and plotted as boxplots as seen in Figure 3. The red line indicates the point at which lactate-46o BP and lactate-80o BP are equal (no effect of 13C-lactate saturation). Some of the brain regions with the highest ratio of lactate-46o BP to lactate-80o BP (atleast two standard deviations above the mean) were the left inferior lateral ventricle, bilateral planum polare, right accumbens area, and left amygdala. In contrast to the right accumbens area, the region with the lowest ratio was the left accumbens area.
Previous studies that have sought evidence of lactate shuttling in the human brain using microdialysis and magnetic resonance spectroscopy have shown that lactate can be oxidatively metabolized to meet energy needs [7, 8]. However, these studies have been limited to a select few regions in the injured brain. The results of this study provide evidence that lactate is converted back to pyruvate for conversion to acetyl-CoA throughout most brain regions.
Conclusion
These results provide in vivo evidence from the human brain that lactate shuttling is occurring at a detectable level: pyruvate is converted to lactate for some non-zero time, back to pyruvate and then to acetyl-CoA for oxidation in the TCA cycle. A related question is whether the observed oxidative metabolism of lactate is occurring in neurons or glial cells and future experiments with diffusion weighting and/or spin labelling may shed light on this question.Acknowledgements
Funding support from the Canadian Institutes for Health Research grant PJT152928.References
[1] Luc Pellerin et al. “Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle”. In: Developmental neuroscience 20.4-5 (1998), pp. 291–299.
[2] George A Brooks. “The science and translation of lactate shuttle theory”. In: Cell metabolism 27.4 (2018), pp. 757–785.
[3] Casey Y Lee et al. “Lactate topography of the human brain using hyperpolarized 13C-MRI”. In: Neuroimage 204 (2020), p. 116202.
[4] Casey Y Lee et al. “Predicting response to radiotherapy of intracranial metastases with hyperpolarized 13C MRI”. In: Journal of Neuro-Oncology 152.3 (2021), pp. 551–557.
[5] Yuankai Huo et al. “3D whole brain segmentation using spatially localized atlas network tiles”. In: NeuroImage 194 (2019), pp. 105–119.
[6] Arno Klein and Jason Tourville. “101 labeled brain images and a consistent human cortical labeling protocol”. In: Frontiers in neuroscience 6 (2012), p. 171.
[7] Clare N Gallagher et al. “The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study”. In: Brain 132.10 (2009), pp. 2839–2849.
[8] Fawzi Boumezbeur et al. “The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy”. In: Journal of Neuroscience 30.42 (2010), pp. 13983– 13991.
Figures