1066
Liver and heart metabolism of [1-13C]-pyruvate in awake and anesthetized rats1A. I. Virtanen Institute, University of Eastern Finland, Kuopio, Finland
Synopsis
Anesthesia has profound effects on overall metabolism, leading to significant signal changes in metabolic MR brain experiments using hyperpolarized [1-13C]pyruvate. In the current study, we studied the possible origins of the signals from rat liver and heart under isoflurane anesthesia and while the animals were awake. Increased metabolite signals, especially lactate, were observed from both organs in awake animals.
Introduction
Hyperpolarized MR allows real-time detection of tissue metabolism, but the interpretation of results can often be difficult. Signals observed in one tissue can be influenced by signals flowing in from elsewhere1. Furthermore, the physiological state of animal, such as fasting2,3, can influence the results. In preclinical research, anesthesia can have profound effects on overall metabolism, leading to significant metabolite signal changes in metabolic MR experiments using hyperpolarized pyruvate4–6. Previously, markedly different lactate levels were observed in the brains of awake and anesthetized rats, but the contribution of systemic effects could not be assessed. In the current study, we investigated cardiac and hepatic signals observed following injection of hyperpolarized [1-13C]pyruvate in isoflurane anesthetized and awake rats to better understand the effects of anesthesia to metabolic MR signals.Methods
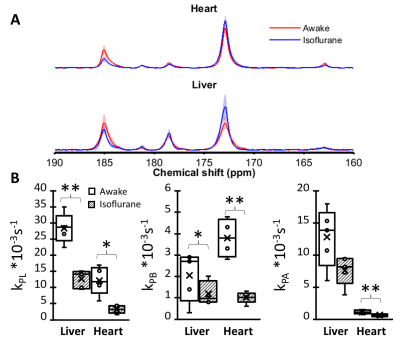
Liver and heart metabolism of hyperpolarized pyruvate was monitored in awake and isoflurane (1.5-1.8%) anesthetized rats. Five female Sprague-Dawley rats (weight 217-268 g) were habituated for awake MR experiments using a four-day protocol described by Stenroos et al. with real MR performed on day 47. Experiment was performed fifteen minutes after the animal had woken up, estimated from increased breathing rate5. 13C experiments were repeated under isoflurane anesthesia three days later. Temperature and breath rates of animals were monitored during the experiments. [1-13C]pyruvic acid was hyperpolarized with radical AH11501 at 1.35 K, 6.7 T and 188 GHz for 1.5 h in experimental HYPERMAG hyperpolarizer (DTU, Denmark). The sample was dissolved with 0.2 M Tris buffer containing 0.1 g/L EDTA and neutralized with 2 M NaOH to yield a ~ 160 mM pyruvate solution with a pH of ~ 7.5. The sample was injected through tail vein (0.8 mmol/kg, 7 ml/min) to an animal inside the magnet. Non-triggered slice-selective 13C spectra (TR=2 s, TE=0.47 ms, nominal flip angle 20°, two 10 mm axial slices centered to liver and heart) were collected at 9.4 T using a home-made 13C transmit/receive surface coil (diameter 4 cm), scout data collected with 1H volume coil (Rapid Biomedical, Rimpar, Germany) was used to slice placing. Only spectroscopy was used because dynamic 13C imaging sequences would have been too loud for awake animals. Peak integrals were estimated and metabolite ratios as well as fits to two-site exchange model were calculated5.Results
As expected, lactate, alanine and bicarbonate resonances were observed in both organs following injection of hyperpolarized pyruvate, although bicarbonate signal was more variable in liver. The lower SNR prevented systematic detection of the other liver metabolites. The relative metabolite signals were significantly larger in awake animals than in isoflurane anaesthetized animals (Figure 1). The bicarbonate-to-lactate ratios were similar in awake and anesthetized animals, ~0.2 and ~0.07 for heart and liver, respectively.Discussion
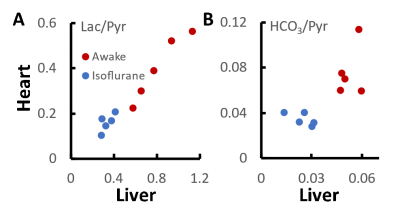
The results show that marked differences in signal labelling are observed between awake and anesthetized animals. Both lactate and bicarbonate were higher in awake animals than in anesthetized animals. It has been previously reported that myocardial lactate labelling is reduced and bicarbonate labelling increased as isoflurane level is lowered6. This raises a possibility that systemic lactate is confounding our spectroscopic experiments as has been reported1. Comparison of heart and liver metabolite levels (Figure 2) suggest a link for lactate but not for bicarbonate. The results also suggest that a significant amount of labelled lactate may be present in blood, and this may have contributed to high lactate levels previously observed in brains of awake rats5. Saturation of in-flowing lactate8 may be therefore essential for non-imaging approaches when awake animals are used.Conclusion
Observed cardiac and hepatic metabolite signals after injection of [1-13C]pyruvate are higher in awake than in isoflurane rats. Some of the cardiac lactate may originate in liver.Acknowledgements
This research was funded by Academy of Finland (grant #332006).References
1. Wespi P, Steinhauser J, Kwiatkowski G, Kozerke S. Overestimation of cardiac lactate production caused by liver metabolism of hyperpolarized [1‐ 13C]pyruvate. Magn Reson Med. 2018;80(5):1882-1890.
2. Jin ES, Moreno KX, Wang J-X, et al. Metabolism of hyperpolarized [1-13C]pyruvate through alternate pathways in rat liver. NMR Biomed. 2016;29(4):466-474.
3. Serrao EM, Rodrigues TB, Gallagher FA, et al. Effects of fasting on serial measurements of hyperpolarized [1-13C]pyruvate metabolism in tumors. NMR Biomed. 2016;29(8):1048-1055.
4. Josan S, Hurd R, Billingsley K, et al. Effects of isoflurane anesthesia on hyperpolarized (13)C metabolic measurements in rat brain. Magn Reson Med. 2013;70(4):1117-1124.
5. Hyppönen V, Stenroos P, Nivajärvi R, et al. Metabolism of hyperpolarised [1– 13 C]pyruvate in awake and anaesthetised rat brains. NMR Biomed. October 2021.
6. Steinhauser J, Wespi P, Kwiatkowski G, Kozerke S. Assessing the influence of isoflurane anesthesia on cardiac metabolism using hyperpolarized [1- 13 C]pyruvate. NMR Biomed. 2018;3(October 2017):e3856.
7. Stenroos P, Paasonen J, Salo RA, et al. Awake Rat Brain Functional Magnetic Resonance Imaging Using Standard Radio Frequency Coils and a 3D Printed Restraint Kit. Front Neurosci. 2018;12:548.
8. Chen AP, Leung K, Lam W, Hurd RE, Vigneron DB, Cunningham CH. Design of spectral-spatial outer volume suppression RF pulses for tissue specific metabolic characterization with hyperpolarized 13 C pyruvate. J Magn Reson. 2009;200(2):344-348.
Figures