1063
Lactate excitation limits bicarbonate detection in hyperpolarized pyruvate MRI of the brain1Aarhus University, Aarhus, Denmark
Synopsis
In hyperpolarized [1-13C]pyruvate MRI of the brain, pyruvate-to-bicarbonate conversion, representing oxidative metabolism, is detected at low levels, despite the highly oxidative metabolism of the brain. We sought to determine the effect of lactate excitation on bicarbonate detection in healthy rats imaged in a cross-over fashion (lactate flip angle = 90° or 0°). In the brain, the bicarbonate signal-to-noise ratio increased 65 % when lactate was not excited. This effect was not observed in the heart, kidneys or liver. Collectively, our data show that lactate saturation limits bicarbonate detection in the brain solely, which has implications for study designs.
Introduction
MRI with hyperpolarized [1-13C]pyruvate enables in vivo detection of glycolysis and glucose oxidation by imaging of the conversion of pyruvate to lactate and bicarbonate. In neuroradiology, the technology yield pathway specific, metabolic information that may show to have diagnostic or prognostic use.1 However, bicarbonate is usually detected at low signal to noise ratios (SNR). In anaesthetized animals, the metabolite is often not detected,2,3 and in human examinations, sensitive hardware is required to detect the metabolite even at low SNR when using dynamic imaging.4 But disregarding bicarbonate means that no information is gained on oxidative metabolism. As dysregulated oxidative metabolism is an important contributor to many neurological diseases,5 the unique potential of hyperpolarized MRI to detect it should not be neglected.A one-cell model is often considered in hyperpolarized MRI (Figure 1); but, in fact, the brain’s metabolism is somewhat compartmentalized as hypothesized in the astrocyte-neuron lactate shuttle model.6,7 In this framework, pyruvate is converted to lactate in astrocytes, shipped to neurons, and metabolized to bicarbonate. Thus, we hypothesized that bicarbonate detection is sensitive to excitation of the lactate resonance, and we aimed to explore if lactate saturation decreases bicarbonate SNR.
Methods
We imaged Sprague Dawley rats on sevoflurane anesthesia (n = 13, 8-week-old males) using hyperpolarized [1-13C]pyruvate on a 3T system (MR750, GE Healthcare) equipped with a 13C/1H-tuned volume coil (RAPID Biomedical). In a cross-over fashion, each rat was scanned twice with an identical sequence, only altering the lactate flip-angle (0° or 90°). In 8 rats, we performed imaging of the brain. Subsequently, we performed multi-slice imaging of the heart (myocardium and blood pool), liver, and kidneys in 5 rats. The sequence employed metabolite-selective excitation (flip angle pyruvate/bicarbonate/lactate = 8°/40°/90° or 8°/40°/0°) with a spiral readout (brain: TR = 500 ms, FOV = 40 mm, matrix = 16⨉16, slice thickness = 16 mm, readout = 24 ms; body: TR = 650 ms, FOV = 80 mm, matrix = 20⨉20, slice thickness = 16 mm, readout = 27 ms). The [1-13C]pyruvate was polarized on a SPINlab polarizer (GE Healthcare). Imaging was initiated at the start of the pyruvate injections (1 ml), which were at least 30 minutes apart. Statistical analyses were performed with linear mixed-effect models.Results
In the brain, bicarbonate SNR increased from 3.55 to 5.81, when lactate was not excited, corresponding to a ~65% gain (Figures 2 and 3). The pyruvate SNR was equivalent between experiments, indicating negligible differences in perfusion or polarization. Likewise, the bicarbonate-to-pyruvate ratio was 0.078 when lactate was saturated versus 0.134 when it was not (Figure 4). In the heart, kidney, and liver, we observed no effects of lactate excitation on bicarbonate SNR (Figure 5).Discussion
Here, we report a considerable increase in bicarbonate SNR when lactate is not excited, yielding bicarbonate clearly and robustly detectable, compared to when lactate is saturated. In extension, we observed that the bicarbonate-to-pyruvate ratio is sensitive to excitation of the lactate resonance. Lastly, this effect seems to be unique to the brain, as we were unable to find the same effect when imaging the heart, kidneys, or liver.Bicarbonate detection is desirable in many neurological conditions such as neurodegeneration or cancer.5 Based on our results, compartmentalized metabolism should be considered in the experimental design depending on the pathway that one desire to investigate. The optimal trade-offs between TR and flip-angles on each metabolite will be the subject for further investigation.
Apart from experimental design, our findings have implications for modeling of hyperpolarized MRI data. The quantification and interpretation of pyruvate-to-bicarbonate metabolism should include the excitation of lactate, which is usually not the case. Further, brain-specific models could consider bicarbonate as a neuronal metabolite, while lactate represents astrocytic metabolism, potentially yielding cell-specific metabolic imaging.
Our findings were made in anaesthetized rats, where detection of bicarbonate is notoriously difficult.2,3 It is likely that the effect of increasing bicarbonate SNR with decreasing lactate excitation is larger in awake humans, as neuronal firing increases pyruvate-to-bicarbonate conversion and astrocyte-neuron coupling.7,8 While bicarbonate is detected in humans with sensitive coils, the increased SNR is still desirable and could be used to improve spatial resolution or decrease the error in model-based quantifications.
Conclusion
In MRI with hyperpolarized [1-13C]pyruvate of the brain, bicarbonate SNR increased ~65% when lactate was not excited, compared to when lactate was saturated. Future studies should consider this effect if robust bicarbonate detection is warranted.Acknowledgements
This work was supported by the Lundbeck Foundation.References
1. Grist, J. T. et al. Hyperpolarized 13C MRI: A novel approach for probing cerebral metabolism in health and neurological disease. J Cereb Blood Flow Metab 40, 1137–1147 (2020).
2. Miller, J. J. et al. 13 C Pyruvate Transport Across the Blood-Brain Barrier in Preclinical Hyperpolarised MRI. Scientific Reports 8, 15082 (2018).
3. Hyppönen, V. et al. Metabolism of hyperpolarised [1–13C]pyruvate in awake and anaesthetised rat brains. NMR in Biomedicine n/a, e4635.
4. Autry, A. W. et al. Characterization of serial hyperpolarized 13C metabolic imaging in patients with glioma. NeuroImage: Clinical 27, 102323 (2020).
5. Chaturvedi, R. K. & Flint Beal, M. Mitochondrial Diseases of the Brain. Free Radical Biology and Medicine 63, 1–29 (2013).
6. Magistretti, P. J. & Pellerin, L. Cellular Bases of Brain Energy Metabolism and Their Relevance to Functional Brain Imaging: Evidence for a Prominent Role of Astrocytes. Cerebral Cortex 6, 50–61 (1996).
7. Mason, S. Lactate Shuttles in Neuroenergetics—Homeostasis, Allostasis and Beyond. Frontiers in Neuroscience 11, 43 (2017).
8. Grieb, B. et al. Curbing action potential generation or ATP-synthase leads to a decrease in in-cell pyruvate dehydrogenase activity in rat cerebrum slices. Sci Rep 11, 10211 (2021).
Figures
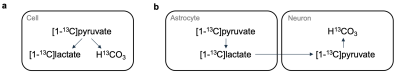
Figure 1: A single-cell model is often considered in MRI with hyperpolarized [1-13C]pyruvate (a). However, in the brain, metabolism is compartmentalized between lactate-producing astrocytes and lactate-consuming neurons (b).
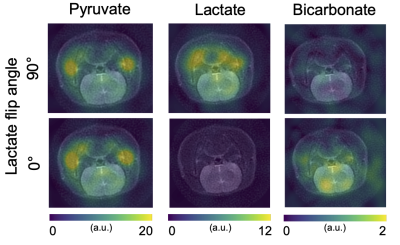
Figure 2: Using dynamic hyperpolarized [1-13C]pyruvate imaging with metabolite selective excitation, saturation of the lactate resonance decreased the bicarbonate signal in the brain of rats.
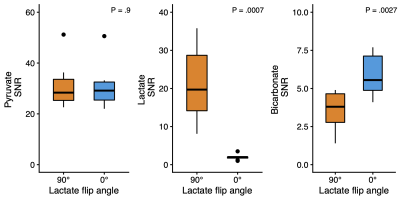
Figure 3: The signal-to-noise ratio (SNR) of pyruvate was constant across experiments, while the lactate SNR expectedly decreased when a flip angle of 0° was used. Interestingly, the SNR of bicarbonate in the brain increased when lactate was not excited.
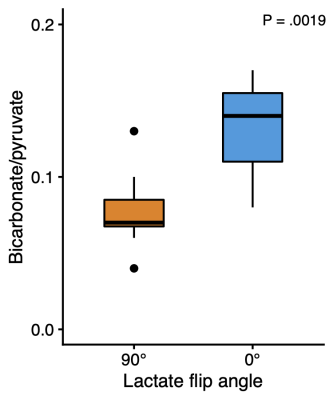
Figure 4: Like the signal-to-noise ratio, a commonly used quantification of pyruvate-to-bicarbonate conversion, their ratio, was also sensitive to lactate excitation.
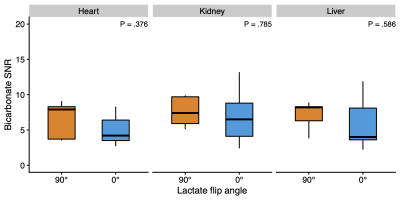
Figure 5: In a subsequent line of experiments (n = 5), we found that the bicarbonate signal-to-noise ratio (SNR) did not change with lactate saturation in MRI with hyperpolarized [1-13C]pyruvate of the heart, kidneys, or liver.