1052
A data-driven method for automatic regularization selection in a hybrid DL-SENSE reconstruction1MR R&D Collaboration, Siemens Medical Solutions USA, Atlanta, GA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 4MR R&D Collaboration, Siemens Medical Solutions USA, Boston, MA, United States, 5Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
The integration of deep learning priors into regularized CG-SENSE reconstructions enables high quality MR images to be generated from noisy, undersampled data. The regularization parameter in these methods can be tuned to control the level of denoising, allowing a network to generalize to novel SNR conditions without retraining. However, manual tuning of the regularization parameter can be time consuming. This work presents a data-driven method for automatic regularization selection using commonly acquired noise calibration data. Results indicate the method generalizes across clinically relevant imaging scenarios and provides diagnostically equivalent image quality to that obtained by manual parameter tuning.
Introduction
Nonlinear image reconstruction methods integrating parallel imaging techniques [1,2] with hand-picked or learned image priors have provided dramatic reductions in imaging time by enabling high-quality images to be reconstructed from noisy, undersampled data [3-9]. Many of these methods include a hyperparameter, called the regularization parameter, that controls the tradeoff between data fidelity and the level of noise/artifact reduction. By tuning this parameter, the same reconstruction algorithm can be adapted to various imaging conditions – a feature which is particularly advantageous when direct adaptation of the image priors is costly. However, manual tuning of the regularization parameter can be time-consuming. Different parameter values may be required for each imaging scenario, and small modifications to the acquisition (e.g., changes in receive coil, resolution, TR/TE, etc.), common in clinical environments, may require additional tuning.While a large body of research has been dedicated to the automatic selection of regularization parameters [10-15], many methods are computationally intensive or make specific assumptions about the image prior. In this work, motivated by the recent success of learning-based methods in image reconstruction [5-9] and regularization parameter selection [14-16], we present a fast, data-driven method for automatic regularization selection in a hybrid DL-SENSE reconstruction [9].
Methods
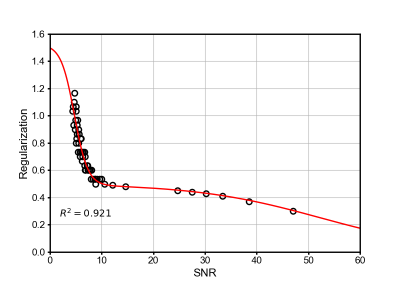
The proposed method learned to select the regularization parameter ($$$\lambda$$$) from an initial estimate of the signal-to-noise ratio (SNR). Specifically, we modeled the mapping from SNR to $$$\lambda$$$ using a sigmoid basis, i.e., $$\lambda(s) = \sum_{\ell=1}^{L} \frac{a_{\ell}}{1+\exp\{-(s-s_{\ell})/b_{\ell}\}} + c,$$ where $$$s$$$ is the SNR, $$$L$$$ is the model order, $$$a_{\ell}$$$, $$$b_{\ell}$$$ and $$$s_{\ell}$$$ are the model parameters, and $$$c$$$ is a normalization constant chosen such that $$$\lambda(\infty) = 0$$$.This model was applied to a hybrid DL-SENSE reconstruction [9], which reconstructed images by solving the following regularized least-squares problem: $$\min_{\rho}||\mathbf{d} - \Omega\mathrm{FC} \mathbf{\rho}||_{2}^{2} + \lambda||\mathrm{WFC}(\mathbf{\rho}_{\mathrm{net}} -\mathbf{\rho})||_{2}^{2},$$ where $$$\mathbf{d}$$$ and $$$\mathbf{\rho}$$$ are the data and image vectors, respectively; $$$\mathbf{\rho}_{\mathrm{net}}$$$ is an initial reconstructed image generated by a trained deep neural network; $$$\Omega$$$, $$$\mathrm{F}$$$, and $$$\mathrm{C}$$$ are the sampling, Fourier transform, and coil-sensitivity operators, respectively; $$$\mathrm{W}$$$ is a pre-determined diagonal weighting matrix chosen to limit the effect of the network prior to unmeasured k-space locations.
The model parameters were determined by fitting the proposed model to training data acquired with various imaging contrasts, undersampling factors, and noise levels using a custom, non-linear optimization routine. For each training dataset, a ground-truth value of $$$\lambda$$$ was determined through manual-tuning, and SNR was computed as the ratio of the mean image intensity to noise standard deviation. Noise standard deviation was computed from a rapid noise-calibration scan routinely acquired for noise decorrelation prior to image acquisition [17]. Mean image intensity was estimated from an initial SENSE reconstruction after foreground segmentation via k-means clustering. Figure 1 shows the resulting model fit with $$$L=2$$$.
Data from two healthy volunteers and five patients with pathologies were acquired on a 3T system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 20-channel head-neck coil and a prototype multi-shot, multi-contrast (T1w, T2w, T2* and FLAIR) acquisition [9] in accordance with the local IRB and HIPAA. Raw data for each contrast were reconstructed once using manually tuned regularization parameters (determined by averaging the preferred values of four expert readers), and once with the auto-selected values. All clinical image pairs were subsequently assessed by two board-certified neuroradiologists, blinded to the underlying reconstruction method.
Results and Discussion
Neuroradiologist evaluation of T1w, T2w, T2*, and FLAIR data indicated that images reconstructed using the automatically selected regularization parameters had diagnostically equivalent image quality compared to images reconstructed using manually tuned regularization parameters. Figure 2 shows representative reconstructions for a patient with cerebral atrophy and findings of chronic cerebral small vessel disease (leukoaraiosis). While it took an average of approximately 1 hour for each of the evaluators to tune the regularization parameters for each contrast, the proposed method took place during the reconstruction itself without a noticeable increase in reconstruction time.The benefit of auto-regularization was particularly evident when protocol parameters deviated from those used during manual tuning. For instance, when regularization parameters manually tuned for acceleration factors R=2 were used for R=3-4, the resulting reconstructions had increased noise, which obscured fine details in the image (Figure 3). Similarly, when the regularization parameter tuned to work with 4 mm thick slices was used with thinner or thicker slices, it led to noisy or overly smooth reconstructions, respectively (Figure 4). In contrast, the proposed auto-regularization method was able to adapt to these protocol changes and provided more consistent image quality in both scenarios.
Conclusion
We proposed a novel, data-driven method for automatic regularization selection in a hybrid DL-SENSE reconstruction. Neuroradiologist evaluation indicated that the method provided diagnostically equivalent image quality to that obtained through time-consuming manual parameter optimization, and results from volunteer data demonstrated that the method could successfully adapt to changes in acquisition parameters.Acknowledgements
The work was funded by the National Institutes of Health (P41EB030006), an RSNA Seed Grant, and research grants from Siemens Healthineers.References
[1] Griswold, M.A., et al., "Generalized autocalibrating partially parallel acquisition (GRAPPA)". Magn Reson Med. 2002;47:1202-1210.
[2] Pruessmann K.P., et al., "SENSE: Sensitivity encoding for fast MRI", Magn Reson Med. 1999;42:952-962.
[3] Liang D, et al., "Accelerating SENSE using compressed sensing: Compressed Sensing SENSE", Magn Reson Med. 2009;62(6):1574-1584.
[4] Mussard E, et al., "Accelerated MP2RAGE imaging using Cartesian phyllotaxis readout and compressed sensing reconstruction", Magn Reson Med. March 2020:mrm.28244.
[5] Hammernik K, et al., "Systematic evaluation of iterative deep neural networks for fast parallel MRI reconstruction with sensitivity‐weighted coil combination", Magn Reson Med. 2021;86(4):1859-1872.
[6] Schlemper J, et al., "A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction", IEEE Trans Med Imaging. 2018;37(2):491-503.
[7] Kim TH, Garg P, Haldar JP. LORAKI, "Autocalibrated Recurrent Neural Networks for Autoregressive MRI Reconstruction in k-Space", arXiv:190409390 [cs, eess]. April 2019. http://arxiv.org/abs/1904.09390. Accessed July 11, 2019.
[8] Eo T, et al., "KIKI‐net: cross‐domain convolutional neural networks for reconstructing undersampled magnetic resonance images", Magn Reson Med. 2018;80(5):2188-2201.
[9] Clifford, B., et al., "Clinical evaluation of an AI-accelerated two-minute multi-shot EPI protocol for comprehensive high-quality brain imaging", in proceeding of International Society of Magnetic Resonance in Medicine (2021).
[10] Hansen, P.C., "The L-curve and its use in the numerical treatment of inverse problems", In: Johnston PR, Ed. Computational Inverse Problems in Electrocardiology. Southampton, UK: WIT Press; 2001:119-142.
[11] Golub, G.H., Heath, M., Wahba, G., "Generalized cross-validation as a method for choosing a good ridge parameter", Technometrics, 1979:21:215-223.
[12] Stein, C.M., "Estimation of the mean of a multivariate normal distribution", Ann Stat. 1981: 9: 1135-1151.
[13] Ramani, S., et al., "Regularization parameter selection for nonlinear iterative image restoration and MRI reconstruction using GCV and SURE-based methods", IEEE Transactions on Image Processing; 2012:21(8):3659-72.
[14] Guan, Y., et al., "Learn to better regularize in constrained reconstruction", in proceeding of International Society of Magnetic Resonance in Medicine (2021).
[15] Peng, X., et al., "Optimal regularization parameter selection for constrained reconstruction using deep learning", in proceeding of International Society of Magnetic Resonance in (2018).
[16] He J., Dong C., Qiao Y. "Modulating Image Restoration with Continual Levels via Adaptive Feature Modification Layers", In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2019:11056-- 11064.
[17] Kellman, P. and McVeigh, E.R., "Image Reconstruction in SNR Units: A General Method for SNR Measurement", Magn Reson Med. 2005; 54:1439-1447.
Figures