1046
Outlier Rejection for Fetal Brain MRS1Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Magnetic resonance spectroscopy (MRS) can measure the concentration of a range of metabolites to understand pathophysiological and normal development in the fetus, as well as provide diagnostic and prognostic markers [1]. However, MRS applications in the fetus are technically very challenging due to the inherently low SNR (fetal size, distance from the coil, the use of 1.5T scans) and signal disruption caused by unrestricted fetal motion during data collection. Given the limited available SNR, outlier rejection to remove motion corrupted averages and improve spectral quality is particularly important. Spectral phase shifts are associated with motion, both during signal acquisition and between MRS repeats [2]. We propose a threshold-free method for optimal outlier rejection of fetal MRS, “Ranked residual-water Phase Shift”: the phase shift in the residual water after water suppression is used as a metric linked to potential motion in individual transients/repeats.
Introduction
Magnetic resonance spectroscopy (MRS) can measure the concentration of a range of metabolites to understand development and pathology in the fetus [1]. However, MRS applications in the fetus are very challenging due to inherently low SNR (fetal size, distance from the coil, 1.5T scans) and signal disruption caused by unrestricted fetal motion. Given the limited available SNR, outlier rejection to remove motion corrupted averages and improve spectral quality is particularly important. Spectral phase shifts are associated with motion [2]. We propose a threshold-free method for optimal outlier rejection of fetal MRS, “Ranked residual-water Phase Shift”: the phase shift in the residual water after water suppression is used as a metric linked to potential motion in individual transients/repeats.Method
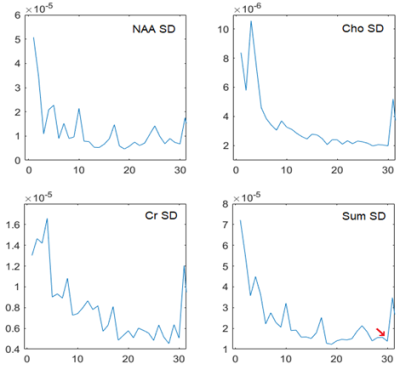
MRS data (PRESS, TE/TR 42/1500ms, 20x20x20mm3, 1.5T Philips Achieva, 128 repeats saved in 32 ‘blocks’ of 4) from 128 fetuses was considered used as a proof-of-concept for the proposed method. Scans of 35 fetuses were selected based on key metabolite peaks being visually identifiable in the mean spectra, with a median gestational age of 29 weeks and range 12.6.Step 1: the phase shift of the residual water peak (~4.65ppm) was estimated for each block of 4 repeats and utilized as the cost metric serving as a surrogate indicator of fetal motion. Due to the magnitude of the water peak - typically still clearly visible in fetal MRS after water suppression - this metric is robust to the low SNR of the fetal data. It is also insensitive to frequency drifts that could be associated with scanner instability or cooling following gradient intense sequences [4]. The relative water peak phase shift - with respect to its phase shift in the first block - was used to rank each block; greater relative shifts assumed to be associated with greater motion ranking lower. Step 2: Frequency and phase correction was applied to data from each block [5] using FID-A [6]. Step 3: Following ranking, an increasing number of blocks of repeats were cumulatively summed in the order of the rank calculated in the first step, i.e. (1st ranked block, 1st and 2nd ranked… all but the last block, all blocks). Step 4: Metabolite concentrations-in each of these sets containing an increasing number of blocks-were quantified with TARQUIN [7] using the default brain basis set. Step 5: The quality metric for each of these fits was the standard deviation of the estimated concentration of the key metabolites - total N-Acetyl-Aspartate (NAA), total Choline (Cho), and total Creatine (Cr) - calculated using Cramér-Rao lower bounds [8]; the averages of these CRLBs was used as single summary metric weighting the contribution of these 3 groups of metabolites according to their concentration.
Without motion, adding more consistent repeats increases SNR, improves fits and reduces concentration uncertainties. However, adding lower-ranked data (with rank linked to difference vs initial data, due to motion) will reduce the quality of the fit, increase estimates uncertainty and warrant the rejection of the corresponding block of repeats (Figure 2). In contrast, the FID-A [6] outlier-rejection function “op_rmbadaverages”, considers the mean square difference of the frequency points between the median spectrum and each repeat, and rejects outliers if this exceeds the standard deviation of all repeats’ sum square differences multiplied by a user defined threshold (NSD). We report the comparison of the proposed “Ranked residual-water Phase Shift” method and the FID-A outlier-rejection method for a number of NSD values.
Results
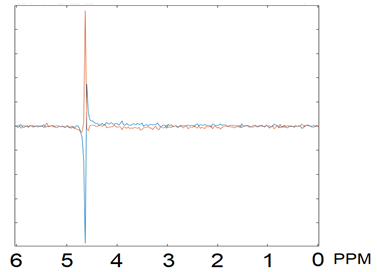
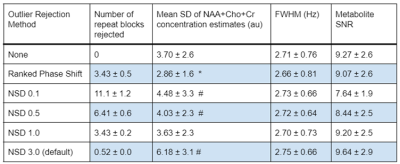
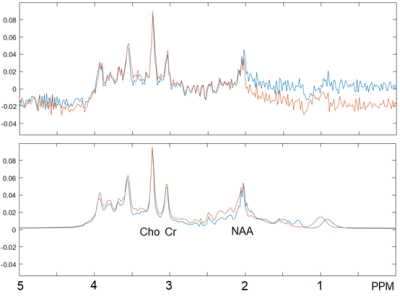
The proposed Ranked residual-water Phase Shift outlier rejection algorithm improved the uncertainty in the quantification of key metabolites relative to no outlier rejection, as indicated by the decreased average metabolite SDs (p = 0.003, Figure 3). It also provides an advantage over the FID-A method. In this dataset FID-A outlier rejection cannot achieve a similar uncertainty reduction irrespective of the chosen NSD, even with a similar number of rejected blocks of repeat as our proposed method. Overall, metabolite linewidths estimated by Tarquin are similar for the different outlier rejection methods. For FID-A, the TARQUIN-estimated SNR decreases with the number of rejected blocks. An example spectrum before and after Ranked Phase Shift outlier rejection is shown in Figure 4.Discussion
Our proposed outlier rejection algorithm for fetal MRS data significantly improved the quantification of key metabolites in a fetal cohort, with no arbitrary prior threshold required. This was achieved by minimising the number of rejected transients, without reducing the quality of the resulting averaged spectrum. Fitting quality (measured as SD) improved, with no significant effects on linewidth and SNR compared to no outlier-rejection or conventional pre-processing. Noise dominates over motion artefact effects in the metabolic area of fetal spectrum (<4.6ppm), but we demonstrate that motion related phase shifts in the residual water peak are readily identifiable and can be used to perform optimised fetal MRS outlier rejection. This method is also consistent with the occasional observation of phase shift in online-displayed residual water peaks during neonatal MRS acquisitions, concurrent with neonatal distress/motion as detected by physiological monitoring or crying. Future work will consider the use of this technique in real time to identify subject motion that may require repositioning of the MRS voxel.Acknowledgements
No acknowledgement found.References
[1] da Silva, Nivaldo Adolfo. Magnetic resonance imaging of the fetal brain at 3 Tesla. Medicine: October 2018[2] MG Saleh. Motion correction in magnetic resonance spectroscopy. MRM. April 2020.[3] Subechhya Pradhan. Magn Reson Imaging. COMPARISON OF SINGLE VOXEL BRAIN MRS AT 3T AND 7T USING 32-CHANNEL HEAD COILS. 2015 Oct.[4] Steve C N Hui. Frequency drift in MR spectroscopy at 3T. Neuroimage. 2021. Nov 1.[5] Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015 Jan;73(1):44-50.[6] Simpson R et al. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magnetic resonance in medicine. 2017 Jan 1;77(1):23-33.[7] Martin M et al. A constrained least-squares approach to the automated quantitation of in vivo ¹H magnetic resonance spectroscopy data. Magn Reson Med. 2011 Jan; 65(1):1-12.[8] Roland Kreis. The trouble with quality filtering based on relative Cramér-Rao lower bounds. MRM. 2015, 06 March.Figures