1044
Dual-encoding of magnetization transfer and diffusion for the characterization of tract-specific myelination1McConnell Brain Imaging Centre, Montreal Neurological Institute and Hospital, Montreal, QC, Canada, 2Department of Biomedical Engineering, McGill University, Montreal, QC, Canada, 3Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 4Hotchkiss Brain Institute and Departments of Radiology and Clinical Neuroscience, University of Calgary, Calgary, Canada, Calgary, AB, Canada
Synopsis
Most white matter voxels are comprised of a combination of microstructurally different tracts that kiss and cross. Conventional MRI contrasts that provide a single measurement per voxel fail to disentangle the microstructural properties of these tracts. Co-encoding diffusion with a complementary contrast offers additional insight into each tract’s individual contribution. Here we present a novel MT-prepared diffusion sequence, optimized for contrast in both MTR and orientation, acquired within a clinically acceptable scan time of under 7 min. The goal is to provide a data acquisition framework for the computation of tract-specific myelin indices and potentially more structurally specific connectomes.
Introduction
Multi-modal MRI can be used to probe complementary tissue properties. Co-encoding contrasts provides the added benefit of colocalization, a potential reduction in scan time, and the ability to isolate compartments based on differences in contrast in one of the modalities (e.g. diffusion), to assess the other contrast (e.g. magnetization transfer (MT)) in that specific compartment. Here, we present a novel MT-weighted diffusion sequence, that simultaneously provides diffusion- and MT- encoded data with the potential to resolve distinct MT properties of any structures with different diffusion orientations; for example tracts that cross or kiss at the sub-voxel level. The acquisition parameters are optimized to co-encode this information in a clinically acceptable scan time.Methods
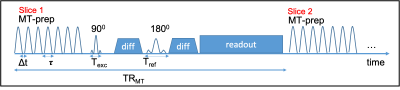
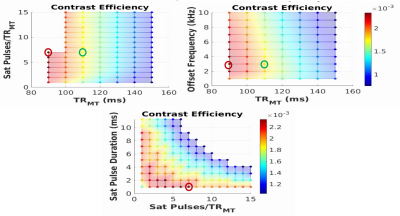
The novel sequence is comprised of a non-selective MT preparation module, inserted prior to the excitation of each slice in a standard diffusion acquisition (Figure 1). Unlike prior implementations using MT-weighted EPI sequences1,2, offset frequency, duration (t), gap (Δt) and number of pulses (single or dual irradiation) as well as the B1rms of the MT module can be controlled by the user at the console.Five dummy scans are included at the beginning of the scan to achieve an MT steady-state. To maximize the MT contrast within the SAR constraints, we reduced the power deposition by avoiding the use of multi-band pulses, and by lengthening and adjusting the ratio of excitation and refocusing pulse durations rather than using a fat saturation pulse for fat suppression3. Removing the fat saturation also removes its unwanted off-resonance contribution. To determine the protocol that maximizes contrast efficiency (MTR/time), simulations of a 3-pool model were carried out in Matlab using the following parameter search space: MT offset frequency=1-10kHz; TRMT=90-120ms; number of pulses=1-15; pulse duration:1-12ms. The following protocol parameters were kept fixed: (Δt=0.3ms, resolution=2.6mm3, 62 slices, TE=58ms, b-value=1500s/mm2, directions=30, Texc=3.328ms, Tref=9.472ms). The B1rms was set to the maximum within the SAR constraints (3.2W/kg for the head). In addition, the total scan time was constrained to a maximum of 10min, which includes the 2 acquisitions, with and without the MT preparation module, needed to compute the MT ratio (MTR =[MToff-MTon]/MToff). Full brain coverage was imposed to allow future use of a global optimization4 framework to estimate bundle-specific MTR values. Two datasets were acquired on a healthy control subject with both an optimal and sub-optimal protocol to validate our simulation results.
Results
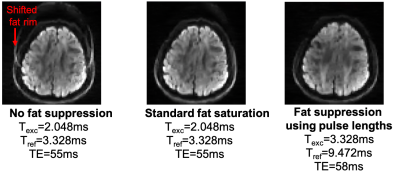
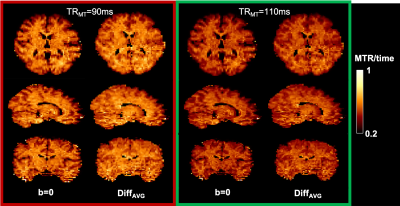
As shown in an example diffusion-weighted image (Figure 2), for the prescribed slice thickness, the fat signal was successfully suppressed with a Texc=3.328ms and Tref=9.472ms at the cost of a slight increase in TE (+3ms) compared to the standard protocol using fat saturation.Based on the simulation results (Figure 3), the optimal protocol is: offset frequency=3kHz; dual irradiation; TRMT=90ms (minimum); number of pulses=7; pulse duration=1ms. These results point to TRMT being the parameter with the most significant impact on MT contrast efficiency. This was verified by acquiring 2 datasets, first using the optimal protocol with TRMT=90ms (red open circle in Figure 3), and second at a higher TRMT =110ms (green open circle) while keeping the total SAR constant at 97% of the allowable limit. This resulted in a B1rms of 7uT and 7.7uT respectively. Figure 4 illustrates the contrast efficiency (MTR/time) of the b=0 image, as well as for the average over 6 of the 30 diffusion orientations (DiffAVG).
Discussion
Our simulation results agree with previous work from Varma et al5, where the use of dual irradiation and short duty cycle help maximize MTR. The optimized protocol can be acquired in under 7min. The primary motivation for this sequence is to be able to provide tract-specific myelin indices, which should in turn lead to more microstructurally specific connectomes. As such, the choice of diffusion parameters (b-value, directions) was motivated by the need to have sufficient contrast between orientations for tractography while maintaining sufficient signal from the extra-cellular compartment. The latter constraint is because the signal from myelin (T2~10-15ms) has mostly decayed at the relatively long TE (50-60ms) required for diffusion EPI sequences, such that the observed MT contrast is thought to primarily arise from the interactions at the surface of the myelin sheaths, and most significantly from the interface with the extra-cellular space. Although the MTR contrast reported here will correlate with myelin volume to some extent, it will be modulated by the surface to volume ratio, sheath thickness, and exchange between compartments, and thus be sensitive to fiber size and packing geometry as well. This optimized acquisition will help explore the virtues of co-encoding MTR and diffusion, by comparing its performance in both voxel-wise (as in6,7) and global tract-based8,9 analysis to methods that use distinct acquisitions for MTR and diffusion contrast (such as tractometry10). Future work will also include exploring model-based corrections for B1+ non-uniformity11 and the reduction of T1 bias in the MTR12.Conclusion
A novel MT-prepared diffusion sequence is presented. Parameters are optimized for MT contrast efficiency through simulations, using a boosted MT saturation method and techniques to reduce the sources of unwanted SAR and off-resonance effects. The resulting protocol can be acquired in under 7 min and represents an important step towards providing tract-specific myelin indices at the sub-voxel level.Acknowledgements
This work was supported by the following funding sources: Quebec Bio-Imaging Network (QBIN), Fonds de Recherche du Québec – Santé (FRQS), and Healthy Brains for Healthy Lives (HBHL).References
1. Gupta RK, Rao AM, Mishra AM, Chawla S, Sekar DR, Venkatesan R. Diffusion-weighted EPI with magnetization transfer contrast. Magn Reson Imaging. 2005 Jan;23(1):35-9. doi: 10.1016/j.mri.2004.11.005. PMID: 15733786.
2. Battiston M, Schneider T, Grussu F, Yiannakas MC, Prados F, De Angelis F, Gandini Wheeler-Kingshott CAM, Samson RS. Fast bound pool fraction mapping via steady-state magnetization transfer saturation using single-shot EPI. Magn Reson Med. 2019 Sep;82(3):1025-1040. doi: 10.1002/mrm.27792. Epub 2019 May 12. PMID: 31081239.
3. Ivanov D, Schäfer A, Streicher MN, Heidemann RM, Trampel R, Turner R. A simple low-SAR technique for chemical-shift selection with high-field spin-echo imaging. Magn Reson Med. 2010 Aug;64(2):319-26. Doi: 10.1002/mrm.22518. PMID: 20574987.
4. Daducci A, Dal Palù A, Lemkaddem A, Thiran JP. COMMIT: Convex optimization modeling for microstructure informed tractography. IEEE Trans Med Imaging. 2015 Jan;34(1):246-57. Doi: 10.1109/TMI.2014.2352414. Epub 2014 Aug 27. PMID: 25167548.
5. Varma, G., Girard, O.M., Mchinda, S., Prevost, V.H., Grant, A.K., Duhamel, G., Alsop, D.C., 2018. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. J. Magn. Reson. 296, 60–71. https://doi.org/10.1016/j.jmr.2018.08.004
6. De Santis S, Barazany D, Jones DK, Assaf Y. Resolving relaxometry and diffusion properties within the same voxel in the presence of crossing fibres by combining inversion recovery and diffusion-weighted acquisitions. Magn Reson Med. 2016;75:372-380.
7. Leppert IR, Andrews DA, Campbell JSW, Park DJ, Pike GB, Polimeni JR, Tardif CL. Efficient whole-brain tract-specific T1 mapping at 3T with slice-shuffled inversion-recovery diffusion-weighted imaging. Magn Reson Med. 2021 Aug;86(2):738-753. doi: 10.1002/mrm.28734. Epub 2021 Mar 21. PMID: 33749017.
8. Simona Schiavi, Marco Pizzolato, Mario Ocampo-Pineda, Erick Canales-Rodriguez, Jean-Philippe Thiran, and Alessandro Daducci. Is it feasible to directly access the bundle’s specific myelin content instead of averaging? A study with Microstructure Informed Tractography. ISMRM 27th Annual Meeting & Exhibition, Montréal, Canada, May 11-16, 2019, #3369.
9. Barakovic M, Tax CMW, Rudrapatna U, Chamberland M, Rafael-Patino J, Granziera C, Thiran JP, Daducci A, Canales-Rodríguez EJ, Jones DK. Resolving bundle-specific intra-axonal T2 values within a voxel using diffusion-relaxation tract-based estimation. Neuroimage. 2021 Feb 15;227:117617. doi: 10.1016/j.neuroimage.2020.117617. Epub 2020 Dec 7. PMID: 33301934.
10. Bells S, Cercignani M, Deoni S, et al. Tractometry comprehensive multi-modal quantitative assessment of white matter along specific tracts. Annual Meeting of the International Society for Magnetic Resonance in Medicine. Montreal, Canada; 2011, #0678.
11. Rowley CD, Campbell JSW, Wu Z, Leppert IR, Rudko DA, Pike GB, Tardif CL. A model-based framework for correcting B1+ inhomogeneity effects in magnetization transfer saturation and inhomogeneous magnetization transfer saturation maps. Magn Reson Med. 2021 Oct;86(4):2192-2207. doi: 10.1002/mrm.28831. Epub 2021 May 6. PMID: 33956348.
12. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med. 2008;60:1396-1407.
Figures