1041
Simultaneous ADC mapping and water-fat separation with B0 correction using a rosette acquisition1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
Synopsis
In diffusion-weighted imaging (DWI) using echo-planar acquisition, fat signals are commonly suppressed by using chemical saturation or by spectral-spatial RF excitation. In this study, we present a technique using a rosette trajectory for simultaneous mapping of apparent diffusion coefficient (ADC) and water-fat separation with inherent B0 correction. The feasibility of using this technique is demonstrated in phantom, brain, and head-neck scans.
Introduction
Diffusion-weighted MRI (DW-MRI) has taken an increasingly important role in probing the tissue microstructure and detecting malignancy of cancer in various parts of the human body, such as the brain1, breast2, prostate3 and abdomen4. The commonly-used readout for DW-MRI is single shot echo planar imaging (SS-EPI), since it avoids significant motion artifacts for its fast speed of collecting k-space data. On the other hand, the use of SS-EPI has a few disadvantages: 1) ghost artifacts introduced by phase mismatch between even and odd echoes; 2) image distortion caused by B0 inhomogeneity; 3) chemical shift artifacts due to the off-resonance of fat signal. Multi-echo acquisition has been used for high-resolution DWI because of the advantages of reduced artifacts and relatively mild distortion. However, chemical shift artifacts are still problematic. There are several methods aimed to address the interference of fat outside EPI, such as chemical shift selective RF pulse5, STIR6 and Dixon method7-9. Chemical shift selective RF pulse techniques are sensitive to B0 inhomogeneity and more demanding to gradient performance. Dixon water-fat separation, which could provide a water-only image and a fat-only image respectively, is widely used in many clinical applications. Recently, Burakiewicz et al10 applied the Dixon method to SS-EPI DWI, the use of three acquisitions at different echo times limits the potential applications of this technique. In this study, we propose an acquisition scheme using a rosette11 trajectory for DWI to enable simultaneous B0 correction, water-fat separation, and ADC mapping in a single scan.Methods
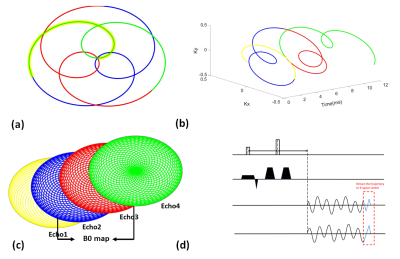
The formulation of rosette k-space trajectory for DWI can be written as follows: $$k(t)=k_{max}sin(w_{1}t)e^{iw_{2}t},$$where $$$w_{1}$$$ and $$$w_{2}$$$ are oscillation and rotational frequency, respectively. The rosette trajectory has a unique property of passing the center of k-space multiple times, it can be used to generate multi-echo images by segmenting the trajectory into different petals. In our experiments, three petals were adopted, the oscillation and rotational frequency were set as 150 Hz and 350 Hz. The scanning parameters are listed as following: TE/TR are 26ms and 3000ms respectively; In-plane resolution is 1.17x1.17 mm2; Slice thickness is 3 mm. Sixty-four rosette shots are used with 1,175 points sampled per shot. The scans use a flip angle of 60 degree and a readout bandwidth of 100 kHz.
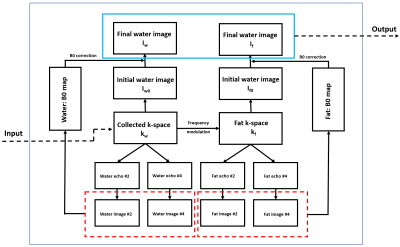
Rosette trajectory has an important spectral property that the signal of on-resonance spins has very little degradation while signal of off-resonance spins could be totally destroyed11. We take the advantage of this spectral property for water-fat separation. The pipeline of water-fat separation and image reconstruction is shown in Figure 2. The frequency modulation is illustrated as follow:
$$k_{f}=k_{w}\cdot conj(\sum_1^6a_{n}e^{-i2\pi f_{n}t}),$$
where $$$f_{n}$$$ represents different frequencies of fat, and $$$a_{n}$$$ is the corresponding amplitudes. $$$k_{w}$$$ represents the collected k-space data of water and $$$k_{f}$$$ represents the k-space data of fat.
All phantom and human subject experiments were conducted on uMR790 3T MR scanner (UIH, shanghai) with 16 channel head neck coils used.
Results
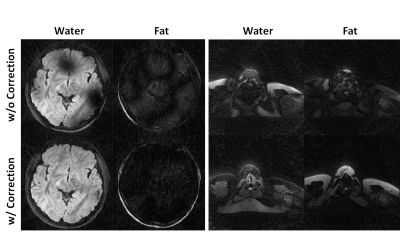
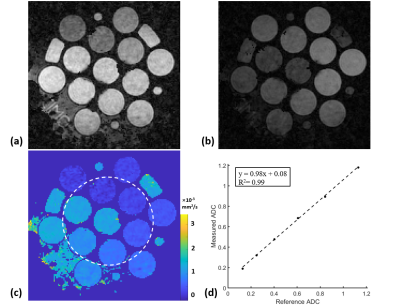
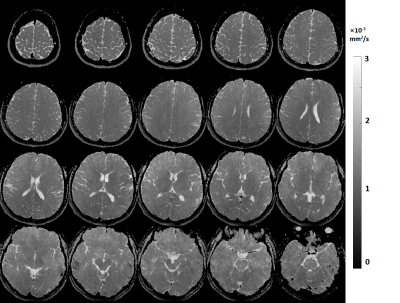
Figure 3 shows that water-fat separation was well achieved with B0 correction in a human subject. Moreover, the robustness of water-fat separation was demonstrated in the neck region where severe B0 inhomogeneity can cause errors in Dixon-based technique. Measured ADC values in Figure 4 agree well with reference value in a standard diffusion model, with a slope of fit line of 0.98 and R2>0.99. Whole brain rosette-DWI was obtained in about 3 minues, as shown in Figure 5. The ADC values measured in several ROIs in white matter agree well with the ones in the literature.Conclusion
The proposed DW-rosette pulse sequence enables simultaneous B0 correction, water-fat separation and ADC mapping in a single scan. The potential clinical applications of this technique remain to be explored.Acknowledgements
We thank Wenlong Feng for experiment preparation and valuable discussion on rosette trajectory design.References
[1] Bihan DL, et al. EMBO Mol Med 2014; 6:569-573.
[2] Amornsiripanitch N, et al. Radiology 2019; 293:504-520;
[3] Lawrence EM, et al. Nat Rev Urol 2012; 9: 94-101;
[4] Tannwald CS, et al. JMRI 2013; 37:35-47.
[5] Meyer CH, et al. MRM 1990; 15:287-304.
[6] Krinsky G, et al. AJR AM J Roentgenol 1996;
[7] Glover GH, et al. MRM 1991; 18:371-383.
[8] Reeder SB, et al. MRM 2005; 54:636-644.
[9] Hernando D, et al. MRM 2010; 63: 79-90.
[10] Burakiewicz J, et al. MRM 2015; 73: 964-972.
[11] Noll DC, et al; IEEE TMI 1997; 372-377.
Figures