1038
3D EPI sequence for indirect detection of hyperpolarized [2-13C]lactate with J-coupling artifact correction1Washington University in St. Louis, St. Louis, MO, United States
Synopsis
Imaging of hyperpolarized (HP) 13C magnetization is typically done using direct detection of the 13C signal. However, indirect 1H-based detection via J-coupled protons has the advantage of reducing gradient requirements and enhancing sensitivity, as well as practical advantages. We have developed an INEPT-based EPI sequence, which we tested by acquiring 3D images of a 13C formate phantom after transfer of thermal polarization from 1H to 13C. We also tested a novel deconvolution method to correct ghosting artifacts introduced in EPI images by J-coupling. These methods will be translated into in vivo HP imaging experiments in the next phase of research.
Introduction
Magnetic resonance imaging of hyperpolarized (HP) 13C agents is a promising technique for assessing metabolic pathways in vivo. A typical HP experiment consists of hyperpolarization followed by direct detection of the 13C signal. Recently, however, indirect detection via polarization transfer (13C to 1H ) has been proposed by multiple groups1–3. Imaging the HP signal on 1H has several advantages, including reduced gradient requirements and increased detection sensitivity3, as well as many practical advantages considering the advanced state of 1H MRI technology. A promising target for polarization transfer is [2-13C]pyruvate, which has a relatively long T1 (~50s)4,5, and produces a metabolite, [2-13C]lactate with a large JCH-coupling constant (~140 Hz)6, allowing for efficient heteronuclear polarization transfer. We have therefore developed a EPI sequence for indirection detection of [2-13C]lactate, as well as a novel method to correct for J-coupling artifacts present in EPI images of [2-13C]lactate.Methods
Sequence developmentTo facilitate sequence development, all testing was performed without HP, and polarization was transferred from 1H to 13C. The first step in our sequence (Figure 1) is VAPOR water suppression7, which in this case serves to suppress the thermal 13C signal. Next is an insensitive nuclei enhanced by polarization transfer (INEPT) block, which omits the traditional 180° refocusing pulses as we placed the signal of interest directly on-resonance. The 90° 1H excitation pulses were 50 μs hard pulses, whereas a 1 ms slice selective sinc pulse was used on 13C. Following the sinc pulse, data was collected during a 3D flyback EPI readout.
In vitro imaging
All experiments were performed on a preclinical 4.7T Varian scanner. A 2 M 13C formate phantom (J = 192 Hz)6 was used as a surrogate for the [2-13C]lactate doublet. To quantify the amount of polarization transfer, we obtained a 1D spectra from a 1 mm coronal slice with and without the 1H hard pulses. To allow for complete relaxation, a TR of 20s was used, and 8 averages were performed. EPI images consisted of a 32x32x4 imaging grid with a 48x48x32mm FOV (1.5 x 1.5 x 8 mm3). The total readout time was 48.64 ms per excitation. Again, a long TR (10 s) was used, and multiple averages (n=16) were acquired. A 3D 1H gradient echo with the same FOV and 96x96x4 grid was also acquired for structural reference.
Correction of J-Coupling Artifacts
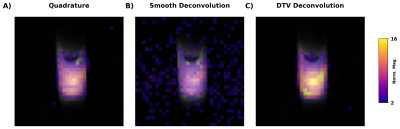
Recently, Datta et al., proposed a quadrature imaging method6 to correct ghosting artifacts in EPI images of J-coupled nuclei (Figure 2). Their method involves combining two datasets acquired 1/2J seconds apart. The requirement for 2 excitations per image is a disadvantage for HP experiments, where hyperpolarization is not renewable and multiple timepoints are often acquired.
An alternative approach is deconvolution. Previous work with 19F MRI has shown that J-coupling artifacts can be eliminated by deconvolving the acquired image with a kernel measured by turning off the imaging gradients8. This kernel would only have to be acquired once at the beginning of the experiment (if no additional peaks appear in the spectra at later timepoints) and should be the same for all slices.
For this study, we compared quadrature correction with deconvolution. As deconvolution of noisy images is not a stable procedure, we tested two different regularization methods. First, we implemented deconvolution with a gaussian smoothness prior9. Second, we used directional total variation (DTV), which constrains the gradients of the deconvolved image to match those of a structural prior10.
Results
A 1D spectra collected from a 1 mm coronal slice through the center of the 13C formate shows the expected 4x signal enhancement with INEPT (Figure 2A). Significant polarization transfer was found in EPI images of the phantom (Figure 2B-C). However, as expected, there was poor spatial correspondence between the 13C EPI and 1H structural images due to J-coupling artifacts (Figure 2C).Consistent with the report by Datta et al.6, we found that quadrature imaging removed the J-coupling artifact without increasing background noise (Figure 3A). Deconvolution with a smoothness prior was successful in removing the ghost, but at the cost of an increase in noise (Figure 3B). In contrast, deconvolution with a DTV constraint was far more stable (Figure 3C).
Discussion
The experiments reported here are the first step in using polarization transfer to acquiredynamic images of HP [2-13C]pyruvate and [2-13C]lactate in vivo. Several limitations of our current design must be addressed before this possible. For HP experiments, we will need to transfer polarization from 13C to 1H, which will require a greater degree of water suppressionas the lactate resonance is very close to the large water resonance. Spectrally selective transfer pulses and partial polarization transfer methods11 will also be needed to permit dynamic imaging, as the standard 90° INEPT hard pulses would use all the available HP after a single acquisition. Finally, we will need to verify that our deconvolution approach is viable in vivo, where limited SNR and incomplete water suppression could represent challenges, especially at later timepoints.
Conclusion
We have successfully developed methods for acquiring EPI images of a JCH-coupled doublet following heteronuclear polarization transfer. More work is needed translate these methods to imaging HP agents.Acknowledgements
Support for these studies was provided by NIH grants S10 OD023580 and R01 DK115987References
1. von Morze, C. et al. In vivo hyperpolarization transfer in a clinical MRI scanner. Magn. Reson. Med. 80, 480–487 (2018).
2. Wang, J. et al. Dynamic 1H imaging of hyperpolarized [1-13C]lactate in vivo using a reverse INEPT experiment. Magn. Reson. Med. 79, 741–747 (2018).
3. Kreis, F., Wright, A. J., Somai, V., Katz-Brull, R. & Brindle, K. M. Increasing the sensitivity of hyperpolarized [15N2]urea detection by serial transfer of polarization to spin-coupled protons. Magn. Reson. Med. 84, 1844–1856 (2020).
4. Park, J. M. et al. Measuring mitochondrial metabolism in rat brain in vivo using MR Spectroscopy of hyperpolarized [2-13C]pyruvate. NMR Biomed. 26, 1197–203 (2013).
5. Chung, B. T. et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. J. Magn. Reson. 309, 106617 (2019).
6. Datta, K. & Spielman, D. MRI of [2- 13 C]Lactate without J-coupling artifacts. 1522–1539 (2021). doi:10.1002/mrm.28532
7. Tkác, I., Starcuk, Z., Choi, I. Y. & Gruetter, R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 41, 649–56 (1999).
8. Busse, L. J., Pratt, R. G. & Thomas, S. R. Deconvolution of chemical shift spectra in two- or three-dimensional [19F] MR imaging. J. Comput. Assist. Tomogr. 12, 824–35
9. Levin, A., Fergus, R., Durand, F. & Freeman, W. T. Deconvolution using natural image priors. Tech Report (CSAIL/MIT) (2007).
10. Ehrhardt, M. J., Gallagher, F. A., McLean, M. A. & Schönlieb, C. B. Enhancing the spatial resolution of hyperpolarized carbon-13 MRI of human brain metabolism using structure guidance. Magn. Reson. Med. 1–12 (2021). doi:10.1002/mrm.29045
11. Norton, V. A. & Weitekamp, D. P. Communication: Partial polarization transfer for single-scan spectroscopy and imaging. J. Chem. Phys. 135, (2011).
Figures
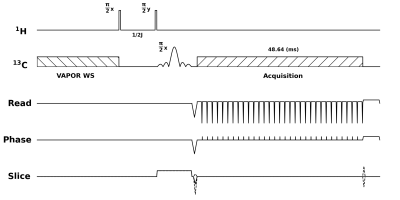
Figure 1: Pulse sequence for 1H to 13C polarization transfer. Sequence began with a VAPOR water suppression module to eliminate thermal 13C signal. This was followed by an INEPT polarization transfer block, consisting of two hard pulses on 1H, and a slice selective sinc pulse on 13C. Finally, a 3D flyback EPI readout was used to acquire a 32x32x4 image.

Figure 2: 1H to 13C polarization transfer using a cylindrical 13C formate phantom. A) A 1D spectra from a 1 mm coronal slice shows, as expected, approximately 4x greater signal with INEPT. Enhancement was also observed when acquiring 3D images of the phantom (B-C). However, as a separate “image” is produced for each peak in the formate doublet, the 13C signal is not restricted to structure of the phantom (in gray). Note that the VAPOR suppression module is turned off for direct detection.