1035
Prospective correction of multi-shot diffusion imaging using motion compensation and dual-speed EPI1CREATIS, Lyon, France, 2Department of Radiology, University Hospital Saint-Etienne, Saint Etienne, France, 3Department of Radiology, Stanford University, Stanford, CA, United States, 4Division of Radiology, Veterans Administration Health Care System, Palo Alto, CA, United States, 5Siemens Healthcare SAS, Saint-Denis, France
Synopsis
Multi-shot (MS) reduces image distortion in EPI caused by B0 field inhomogeneity and enables higher resolution diffusion imaging. However, interaction between physiologic motion and diffusion encoding gradients leads to strong image aliasing artifacts in MS-EPI due to phase discrepancies. We propose prospective strategies to reduce shot-to-shot phase variation for interleaved phase-segmented diffusion MS-EPI. First and second order motion compensated diffusion gradient waveforms and dual-speed EPI with oversampled k-space center were evaluated as a shot-to-shot phase mitigation strategy. Validations were performed in silico, in vitro, and in vivo in the brain, liver, and heart.
Introduction
Diffusion weighted imaging (DWI) is widely used to probe the displacement of water molecules within the tissue. DWI is usually acquired using a single-shot spin-echo echo pIanar imaging (SS-EPI) with diffusion encoding gradients placed symmetrically on either side of the 180° refocusing pulse. In this traditional implementation, DWI has been robustly applied to several organs.Despite, its speed and efficiency, SS-EPI images remain severely affected by B0 inhomogeneities, which create image distortion artifacts in the phase encoding direction. One way to reduce this B0 sensitivity is to shorten the length of the EPI echo train (TEpi) by reducing the number of k-space lines acquired in the EPI readout. High readout bandwidth, partial Fourier, and parallel imaging all reduce TEpi, but at the expense of a lower signal-to-noise ratio (SNR).
Multi-shot spin-echo EPI (MS-EPI), in which k-space is acquired segmentally, results in a lower number of k-space lines acquired per shot, hence a shorter TEpi. However, MS-EPI suffers from shot-to-shot phase variations due to the interaction between physiologic motion and the diffusion encoding gradients1,2. These phase variations generate strong image aliasing artifacts. Several retrospective methods have been proposed3,4, such as low-rank constrained approaches, However these retrospectives strategies remain impractical as they require long, offline reconstruction processes and are subject to extensive ad hoc tuning.
In this work, we propose prospective strategies to reduce shot-to-shot phase variation for interleaved phase-segmented MS-EPI. First (M0M1) and second order (M0M1M2) motion-compensated gradient waveforms have been used in cardiac diffusion imaging to limit the impact of cardiac motion on the diffusion signal5,6. The first objective of this work was to investigate motion-compensated waveforms to reduce shot-to-shot phase variation compared to traditional monopolar encoding (M0). The second objective was to evaluate a new phase-segmented EPI k-space sampling strategy, in which the center of k-space is fully sampled with each shot. This sampling pattern involves two speeds of EPI phase encoding and few extra k-space lines. In silico, in vitro, and in vivo validations on the brain, liver and heart are proposed.
Methods
All analyses proposed in this work were based on a protype DWI sequence (FOV=200x200mm2, matrix=128x128, Partial Fourier=6/8 slice thickness=5mm, Bandwidth=2056Hz/px TR=2000ms). One b-value=0 image and six diffusion-weighted directions were used with b-value=800s/mm2 for brain and in vitro experiment, b-value=500s/mm2 for liver and b-value=350s/mm2 for heart. All comparison were done at a constant acquisition time (2min) by adjusting the number of averages (Navg=8,4,2,1) for the corresponding number of shot (Nshot=1,2,4,8). TE was minimized for a given Nshot and a given diffusion encoding waveform.The dual-speed EPI readout was built by changing the phase encoding blip amplitude and by adding extra lines at the center of the k-space as shown in Figure 1. The number of extra lines was +8 for Nshot=2 and +16 for Nshot=4,8. TEpi was then longer for dual-speed EPI than single speed EPI at equivalent Nshot.
In silico simulations using a Shepp-logan phantom were performed to study shot-to-shot phase variation and B0 inhomogeneities. Single and dual-speed EPI with Nshot=1,2,4,8 were compared.
In vitro and in vivo experiments were performed on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). First B0 inhomogeneities were studied by using a phantom composed of seven tubes with different diffusion coefficients and a tube with trapped air. Then, three healthy volunteers (N=3) were imaged using single and dual-speed EPI with Nshot=4. Diffusion encoding waveform M0, M0M1 and M0M1M2 were compared in the brain and liver while only M0M1M2 was acquired on the heart. All reconstructions were performed offline. GRAPPA with 24 external reference lines was used as an unaliasing technique on each individual shot.
Results
In silico analysis (Figure 2) shows image aliasing due to shot-to-shot phase variation. Image aliasing increases as the number of Nshot increases. GRAPPA reconstruction can unalias images at Nshot=2, but cannot recover highly aliased images at Nshot=4,8. Dual-speed EPI mitigates aliasing and enables unwrapped images with GRAPPA even for Nshot=4,8. Lower root-mean-square-error (RMSE) were found for dual-speed EPI compared to single-speed.As shown in Figure 3, in silico and in vitro B0 inhomogeneity experiments show a decrease of image distortion as the number k-space lines/shots decrease. Since its Tepi is longer, dual-speed EPI yields more image distortion than single-speed EPI for equivalent Nshot. Nonetheless, lower RMSE was achieved with dual-speed EPI compared to SS-EPI.
Examples of in vivo acquisitions for brain, liver and heart are shown in Figure 4. M0M1M2 motion-compensated diffusion encoding waveforms are sufficient to mitigate shot-to-shot phase variation in brain even using single-speed EPI. For the liver and heart acquisitions, only dual-speed EPI led to non-aliased images. Equivalent MD values were found between single-speed and dual-speed EPI across organs (Figure 5). Dual-speed EPI with M0M1M2 leads to lower FA in gray matter and a higher FA in white matter than single-speed EPI. Dual-speed EPI also significantly increases SNR in the brain and liver.
Discussion and conclusion
In this work, M0M1M2 waveforms were shown to mitigate shot-to-shot phase variation for MS-EPI diffusion imaging in the brain. Dual-speed EPI offers a tradeoff between B0 inhomogeneity and aliasing artifacts mitigation. Dual-speed EPI enabled MS acquisitions in moving organs like in the liver and the heart.Acknowledgements
No acknowledgement found.References
1. Bammer R. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 2003;45:169–184 doi: 10.1016/S0720-048X(02)00303-0.
2. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition: EPI With Parallel Imaging and 2D Reacquisition. Magn. Reson. Med. 2009;62:468–475 doi: 10.1002/mrm.22024.
3. Hu Y, Levine EG, Tian Q, et al. Motion-robust reconstruction of multishot diffusion-weighted images without phase estimation through locally low-rank regularization. Magn. Reson. Med. 2019;81:1181–1190 doi: 10.1002/mrm.27488.
4. Mani M, Jacob M, Kelley D, Magnotta V. Multi-Shot Sensitivity-Encoded Diffusion Data Recovery Using Structured Low-Rank Matrix Completion (MUSSELS). Magn. Reson. Med. 2017;78:494–507 doi: 10.1002/mrm.26382.
5. Gamper U, Boesiger P, Kozerke S. Diffusion imaging of the in vivo heart using spin echoes–considerations on bulk motion sensitivity. Magn. Reson. Med. 2007;57:331–337 doi: 10.1002/mrm.21127.
6. Stoeck CT, Deuster C von, Genet M, Atkinson D, Kozerke S. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn. Reson. Med. 2016;75:1669–1676 doi: 10.1002/mrm.25784.
Figures
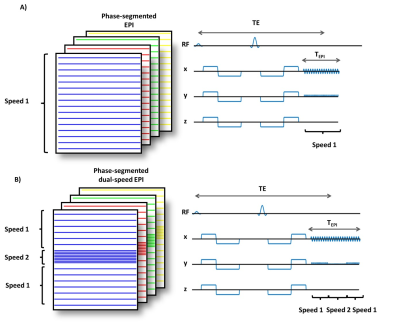
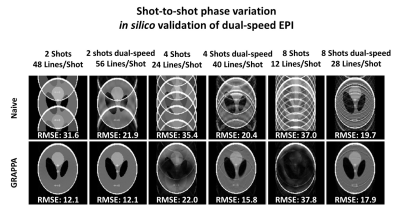
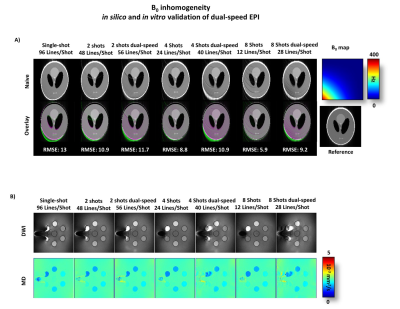
Figure 3: In silico and in vitro comparison of multi-shot dual-speed and single-speed EPI for Nshot=1,2,4,8. A) A distortion is induced in silico by adding an off-resonance gradient. B) Off-resonance was generated in vitro by adding a tube of air. Distortion decreases as the number of lines/shot decreases thus dual-speed EPI yield more distortion than single-speed EPI at equivalent Nshot.
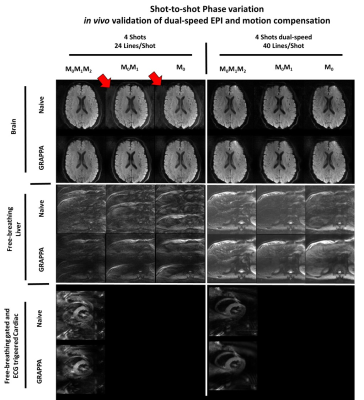
Figure 4: In vivo comparison of multi-shot dual-speed and single-speed EPI for Nshot=4 with motion-compensated diffusion encoding in the brain (first block), liver (second block) and heart (third block). For brain imaging, M0M1M2 waveforms mitigate phase variation while aliasing is present for the other waveforms as noted by the red arrows. For liver and heart which have more consequent motion, dual-speed EPI and M0M1M2 waveforms were necessary to mitigate aliasing.
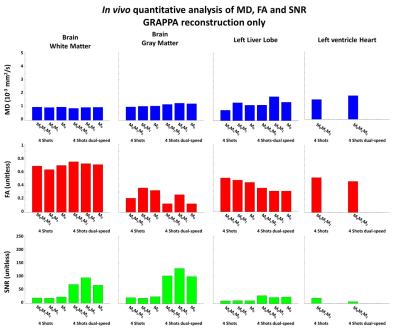
Figure 5: In vivo comparison of Mean diffusivity (MD), Fraction of anisotropy (FA) and signal-to-noise rate (SNR) across volunteer (N=3). Multi-shot dual-speed and single-speed EPI using Nshot=4 with motion-compensated diffusion encoding were compared using GRAPPA reconstruction in the brain, liver, and heart. Overall dual-speed EPI had higher SNR in the brain and in the liver, but a smaller SNR was found in the heart.