1033
Simultaneous J-difference editing of GABA and GSH with extended echo-times using ExTE-HERMES1Physical Sciences Research Platform, Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
J-difference editing techniques, such as MEGA-PRESS, are often used to detect gamma-aminobutryic acid (GABA) and glutathione (GSH). However, two separate scans are needed to acquire both metabolites. The HERMES editing scheme does address this issue, allowing simultaneous acquisition of GABA and GSH. Even then, there are limitations as the optimal echo time (TE) can only be set for one metabolite at the expense of lower editing efficiency for the other. We propose the Extended TE (ExTE) HERMES editing scheme, which allows us to acquire edited spectra at TE=120ms, ensuring maximum GSH signal while still providing efficient GABA editing.
Introduction
Gamma-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the brain and glutathione (GSH) is the primary antioxidant. Due to their low concentration, they are obscured by larger metabolite resonances, such as creatine and NAA. Thus, J-difference editing techniques, such as MEGA-PRESS1, are often used to detect these metabolites. MEGA-PRESS employs a pair of frequency selective RF editing pulses to refocus a coupling partner of the target resonance (1.88 ppm for GABA and 4.56 ppm for GSH) which in turn refocuses the target resonances at around 3.0 ppm. However, due to the differing editing schemes, two separate scans are needed to acquire both metabolites.This is addressed by the HERMES sequence2, which can simultaneously acquire both edited GABA and GSH in a single acquisition. This involves four interleaved experimental conditions: applying editing pulses to both GABA and GSH, editing only GABA, editing only GSH, and editing OFF. The resulting subspectra are then combined using a Hadamard reconstruction which results in either a GABA- or GSH-edited spectrum. One limitation of HERMES is that the optimal echo time (TE) can only be set for one metabolite at the expense of lower editing efficiency for the other. As a compromise, TE is typically set at 80ms, which is neither ideal for GABA nor GSH, which have maximal editing efficiencies at TE=68ms and TE=120ms3, respectively.
Previously, Extended TE (Ex-TE) MEGA-PRESS has shown the possibility of acquiring J-difference edited GABA spectra at longer TEs while maintaining optimal editing efficiency4. This is achieved by applying editing pulses with adjusted timing instead of the conventional edit-OFF acquisition. Specifically, for the “J-inverted” (inv) scan, the editing pulses are applied (TE-1/2J)/2 apart, yielding maximal inversion of the C4-GABA resonance. During edit ON, or the “J-refocused” (ref) scan, the pulses are TE/2 apart.
Here, we propose to combine the ExTE concept with HERMES to enable optimal GSH editing efficiency with a TE of 120ms while still providing efficient GABA editing.
Methods
The HERMES and ExTE-HERMES pulse sequences are shown in Figure1 and Figure2. The ExTE-HERMES sequence is composed of four experiments: A) a simultaneous “GABA and GSH refocused” scan (editing at 1.88 ppm and 4.56 ppm, respectively) using dual-band editing pulses with TE/2 spacing; B) a simultaneous “GABA refocused and GSH inverted” scan (editing at 1.88 ppm and 7.5 ppm, respectively) using dual-band editing pulses with TE/2 spacing; C) a sequential “GABA inverted and GSH refocused ” scan using single-band editing pulses at 1.88 ppm and 4.56 ppm with (TE-68ms)/2 and TE/2 spacing, respectively; D) a sequential “GABA and GSH inverted” scan using single-band editing pulses at 1.88 ppm and 7.5 ppm with (TE-68ms)/2 and TE/2 spacing, respectively.Simulations: HERMES and ExTE-HERMES sequences were simulated with GABA and GSH spin systems using the FID-A processing toolbox (GitHub.com/CIC-methods/FID-A). HERMES was simulated with TEs of 80ms and 120ms, while ExTE-HERMES was simulated with a TE of 120ms. Both sequences were simulated with the following parameters: spectral width = 2000 Hz; number of points = 2048; editing pulse duration = 14 ms.
Phantom Scans: A 3T Siemens Prisma-XR (Erlangen, Germany) was used to perform HERMES and ExTE-HERMES scans on a 500mL phantom containing 50mM GABA and 50mM GSH in water (pH = 7.1). 14ms Gaussian shaped single- and dual-banded editing pulses were utilized. Parameters used for both scans were: TR = 2400ms; spectral width = 2000 Hz; number of points = 2048; number of averages = 32. HERMES sequences were run with TE=80ms and TE=120ms, and ExTE-HERMES was run with TE=120ms.
Processing: All processing was performed using FID-A. Peak integration was used to estimate GABA-edited and GSH-edited peak areas from 2.5 to 3.25 ppm.
Results
Simulation results for GABA and GSH are displayed on Figure3. ExTE-HERMES, HERMES (TE=80ms), and HERMES (TE=120ms) gave GABA peak areas of 1.97, 2.04, and -0.58, respectively (Figure5A). For GSH, ExTE-HERMES, HERMES (TE=80ms) and HERMES (TE=120ms) had peak areas of 3.03, 2.88, and 1.32, respectively.Phantom scan results are shown in Figure4. GABA peak areas of 5.47, 8.12, and -3.40 were calculated for ExTE-HERMES and HERMES with TE=80ms and TE=120ms, respectively (Figure5B). With GSH, ExTE-HERMES had a larger peak area of 9.90 compared to HERMES with TE=80ms and TE=120ms at 4.34 and 6.34, respectively.
Discussion
ExTE-HERMES enables optimal J-difference editing efficiency of both GABA and GSH signal at TEs well above the normal ideal range for GABA. Compared with HERMES (TE=80ms), phantom scans show a decreased GABA peak area using ExTE-HERMES, which could be due to T2 decay. However, ExTE-HERMES shows a significant increase in GSH signal compared to both HERMES scans. Further investigation is needed to understand this signal increase.This sequence can be adapted to other metabolites as well, opening the door for J-difference editing of metabolites with differing TE conditions. Due to the longer TE used, MM resonances are attenuated by virtue of their short T2, resulting in reduced MM contamination. One limitation of ExTE-HERMES is that the sequence is restricted to a minimum TE of ~120 ms due to the widths of the editing pulses, crusher gradients, and refocusing pulses..
Future work will involve the acquisition of in-vivo data to further assess the benefits of ExTE-HERMES.
Acknowledgements
No acknowledgement found.References
[1] Mescher, M., Merkle, H., Kirsch, J., Garwood, M., & Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine, 11(6), 266–272. https://doi.org/10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j
[2] Saleh, M. G., Oeltzschner, G., Chan, K. L., Puts, N., Mikkelsen, M., Schär, M., Harris, A. D., & Edden, R. (2016). Simultaneous edited MRS of GABA and glutathione. NeuroImage, 142, 576–582. https://doi.org/10.1016/j.neuroimage.2016.07.056
[3] Chan, K. L., Puts, N. A., Snoussi, K., Harris, A. D., Barker, P. B., & Edden, R. A. (2017). Echo time optimization for J-difference editing of glutathione at 3T. Magnetic resonance in medicine, 77(2), 498–504. https://doi.org/10.1002/mrm.26122
[4] Near, J., Kumaragamage, C. (2015, May 30 - June 5). J-difference editing of GABA with extended echo times. [Poster presentation] 23rd ISMRM Annual Meeting & Exhibition, Toronto, ON, Canada.
Figures
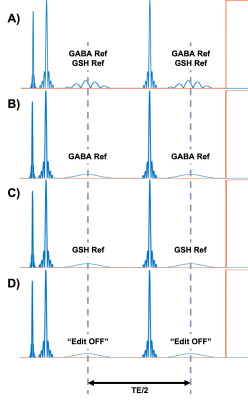
Figure1: Editing scheme for HERMES: A) “GABA and GSH refocused” scan (editing at 1.88 ppm and 4.56 ppm, respectively) using dual-band editing pulses; B) “GABA refocused” scan using single-band editing pulses at 1.88 ppm; C) “GSH refocused” scan using single-band editing pulses at 4.56 ppm; D) “Edit OFF” scan using single-band editing pulses at 7.5 ppm. Editing pulses are set TE/2 apart from one another to ensure efficient metabolite refocusing.
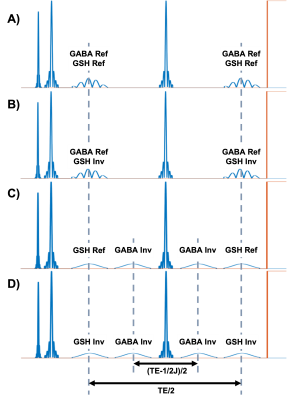
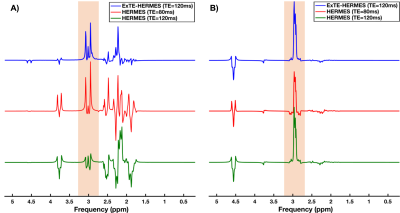
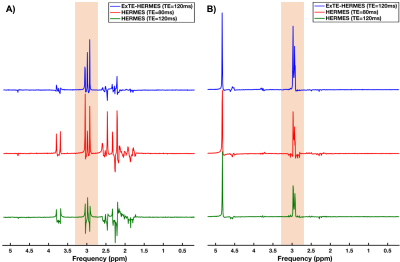
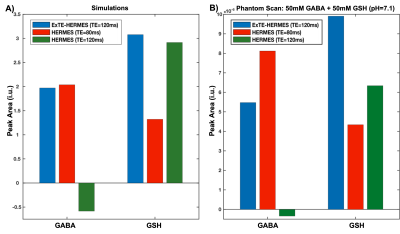
Figure5: GABA and GSH peak areas calculated from the simulated (A) and phantom (B) scans. A) Simulated GABA peak areas: 1.97 (ExTE-HERMES); 2.04 (HERMES TE=80ms); and -0.58 (HERMES TE=120ms). Simulated GSH peak areas: 3.08 (ExTE-HERMES); 1.32 (HERMES TE=80ms); and 2.92 (HERMES TE=120ms). B) Phantom GABA peak areas: 5.47 (ExTE-HERMES); 8.12 (HERMES TE=80ms); and -3.40 (HERMES TE=120ms). Phantom GSH peak areas: 9.90 (ExTE-HERMES); 4.34 (HERMES TE=80ms); and 6.34 (HERMES TE=120ms).