1016
Multimodal apparent diffusion model provides comprehensive analysis of clinical subacute stroke lesions1Radiology, University of Illinois at Chicago, Chicago, IL, United States, 2Medical Imaging, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China, 3Pathology, University of Illinois at Chicago, Chicago, IL, United States, 4Radiology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China, 5Biomedical Engineering, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
We present our initial findings of applying Multimodal Apparent Diffusion (MAD) MRI, including b-values up to 10,000 s/mm2, to analyze subacute stroke lesions for the characterization of stroke lesions based on micro-environment biomarkers in order to guide treatment in clinics. MAD model is able to separate the acquired diffusion weighted signal into flow, and, unimpeded (fluid), hindered, and restricted apparent diffusion components. Significant differences were found in MAD parameters between lesions and normal appearing white matter. Fraction of unimpeded diffusion significantly increased. Hindered diffusion was significantly lower in lesions. On the other hand, restricted diffusion was significantly higher.
Introduction
Ischemic stroke is the second most common cause of death and the leading cause of acquired disability in adults[1,2]. Biomarkers are needed to reflect relevant events in the ischemic cascade, from cellular bioenergetic failure, through excitotoxicity, oxidative stress, blood–brain barrier dysfunction, microvascular injury, hemostatic activation, post-ischemic inflammation and finally cell death of neurons, glial and endothelial cells[3]. There is a need to improve the accuracy of stroke diagnosis and to more reliably predict stroke outcome. Diffusion weighted MRI has long been used to detect the onset of ischemic stroke. Albeit, there is still the quandary as to when the ischemic cascade has progressed to the point that recanalization would be ineffective and possibly damaging or lethal. Herein we present our initial findings of applying Multimodal Apparent Diffusion (MAD) MRI [4], including b-values up to 10,000 s/mm2, to analyze subacute stroke lesions in order to characterize stroke lesions based on micro-environment biomarkers for the treatment guidance. MAD methodology separates the acquired diffusion weighted signal from low to high b values into flow, and unimpeded (fluid), hindered, and restricted apparent diffusion components, providing their fractions and diffusion coefficients.Methods
In an IRB approved study, 41 patients were imaged with confirmed subacute stroke (25m/16f, 68±12yo) with a total of 324 lesions. MAD images were acquired on a Siemens/Prisma 3T MRI scanner with b-values (0/1, 500/1, 1000/1, 2000/2, 3000/2, 4000/3, 6000/3, 8000/4, 10,000/6 s/mm2 /averages) and acquisition/image matrix 128x128/256x256, FOV 230x230x5 mm, TR/TE 3900/69 ms, with an acquisition time of 3.7 min. Diffusion weighted images were analyzed with the comprehensive MAD method [4], which separated the apparent diffusion contrast into, flow (>> 3 µm2 /ms), unimpeded diffusion (3 µm2/ms), hindered diffusion (> 0.1 & < 3 µm2/ms), and restricted diffusion (< 0.2 µm2/ms). ROIs were drawn based on the elevated signal on the b=1000 s/mm2 image. ROIs were also drawn around normal appearing white matter (NAWM). Student’s t-test was used to compare the NAWM ROIs’ means with the means of lesion ROIs’ with predominately homogeneous responses (n=238) the Hindered and Restricted diffusion coefficients by using p < 0.05 as statistical significance.Results
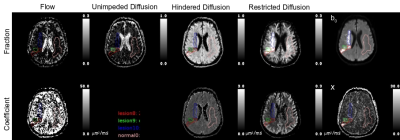
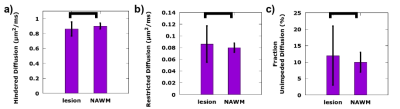
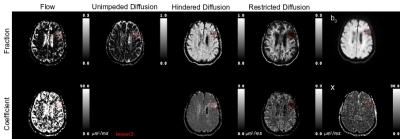
Figure 1 presents a example of the MAD model parameters for lesions with predominately homogeneous MAD parameter contrast. There were number of lesions (n=86) with multiple regions of homogeneous MAD parameter contrast, see figure 2 for an example. The lesions’ hindered diffusion coefficients were significantly lower than that of NAWM and with a wider variation, 0.86±0.095 µm2/ms, 0.90±0.040 µm2/ms, respectively, p<0.01, figure 3. The lesions’ restricted diffusion coefficients were significantly larger than that of NAWM and with a wider variation, 0.086±0.031 µm2/ms, 0.080±0.008 µm2/ms, respectively, p=0.01. The lesions’ fractions of unimpeded diffusion (fluid) were significantly higher than that of NAWM and with a wider variation, 12±9 percent, 10±3 percent, respectively, p<0.01.Discussion
The reduced hindered diffusion coefficient in the stroke lesions are consistent with cytotoxic edema associated with cellular bioenergetic failure due to ischemia. The wider variation across the lesions may be reflective of the varying degrees of ischemic cascade progression. The lowering of the restricted diffusion coefficient in lesions may be due to the excitotoxicity and coagulative necrosis expelling the cellular contents into the extracellular space. The multiple homogenous restricted diffusion regions within heterotypic lesions may be reflective of the neutrophils and/or macrophages processing the cellular debris, figure 2. The increase in the amount of unimpeded diffusion (fluid) and the increased hindered diffusion coefficient in the lesions are consistent with increased inflammation and necrosis, figure 4.Conclusion
By analyzing a wide spectrum of apparent diffusion covering b-value from low to ultrahigh, multimodal apparent diffusion MRI helps to characterize clinical subacute ischemic lesions. Investigations are ongoing for further validations.Acknowledgements
No acknowledgement found.References
[1] Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: GlobalBurden of Disease Study. Lancet 1997;349:1269–76.
[2] Donnan GA,Fisher M,Macleod M,Davis SM. Stroke. Lancet 2008;371:1612–23.
[3] Brouns R, De Deyn PP, The complexity of neurobiological processes in acute ischemic stroke. Clinical Neurology and Neurosurgery 111 (2009) 483–495
[4] Damen FC, Scotti A, Damen FW, Saran N, Valyi-Nagy T, Vukelich M, Cai K. Multimodal apparent diffusion (MAD) weighted magnetic resonance imaging. Magn Reson Imaging. 2021 Apr;77:213-233. doi: 10.1016/j.mri.2020.12.007. Epub 2020 Dec 10. PMID: 33309925; PMCID: PMC7878401.
Figures