1015
Serial in vivo 19F MRI tracks the immune cell response to bioscaffolds implanted in a rat model of stroke1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 3Biological Science, University of Pittsburgh, Pittsburgh, PA, United States, 4Surgery, University of Pittsburgh, Pittsburgh, PA, United States, 5Neurobiology, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
Regenerative medicine is increasingly investigating how an initial pro-inflammatory response convert to a pro-repair response. Implantation of bioscaffolds exploits this mechanism and allows tissue to regrow in large defects. Even the brain, which is known to not regrow tissue spontaneously, can regenerate lost tissue after implantation of a inductive bioscaffold. Little is known about how immune cells respond to the implantation of a bioscaffold. We here characterized the acute spatiotemporal dynamics of immune cell infiltration into the brain and a bioscaffold by tagging immune cells with perfluorocarbon nanoemulsions for detection by 19F MRI and anatomical context using 1H MRI.
Methods:
Animals. Sprague-Dawley rats (12 weeks old) underwent 70 minutes of right middle cerebral artery occlusion to model stroke. Twelve days post-stroke animals were injected with perfluorcarbon (PFC) nanoemulsions (360 mg/mL, V-Sense, CelSense, Pittsburgh, PA) through the tail vein to label circulating immune cells. 19F MRI acquisition parameters were previously optimized for the fast imaging with steady-state precession (FISP) sequence(2). A baseline 19F (TR=4.8 ms, TE=2.4 ms, matrix 96x96, FOV 40 x 40 mm, slice thickness 1.5 mm, acquisition time 60 minutes) and geometrically identical T2-weighted (RARE, TR=5000 ms, TE=40 ms, matrix 192x192, slice thickness 1.5 mm, acquisition time 60 minutes) 1H MRI scan was acquired to verify the delivery of PFC, as evidenced by “hot” blood vessels. Thirteen days post-stroke ECM hydrogel (4 mg/mL) was injected into the stroke cavity. 19F MR images were acquired at 1, 2, 3, 4, 5, 6, 9, 18, and 24 hours post-implantation. 19F spins were quantified for the peri-infarct area and the ECM hydrogel using VoxelTracker (CelSense). After scanning, rats were perfused. Fluorescence immunohistochemistry on brain tissue quantified the number of macrophages, lymphocytes and neutrophils that invaded the peri-infarct area and the ECM hydrogel. Co-localization with the red fluorescence of the PFC afforded a quantitation of the number of PFC-labeled and -unlabeled cells.
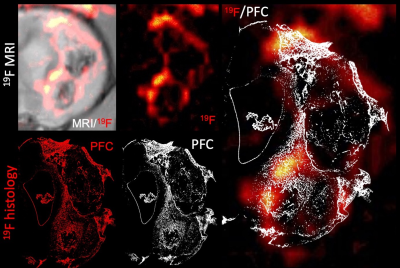
Results: Baseline 19F/1H MR images revealed the administration of PFC nanoemulsion into the blood stream with no PFC leaking into the stroke lesion (12 days post-stroke). Upon implantation of an ECM hydrogel into the stroke cavity, a major signal increase in 19F was evident in the peri-infarct area, as well as the ECM hydrogel (Figure 1A). Quantitation of the signal over time indicated an increased signal first in the peri-infarct tissue at 6 hours post-implantation, followed by a gradual invasion into the ECM hydrogel peaking at 12 hours post-implantation (Figure 1B). Histological verification of the PFC-labeled cells distribution validated that the 19F signal reflects the infiltration of immune cells (Figure 2). Immunohistochemistry further indicates that only a very small proportion of immune cells (1-2%) in the peri-infarct area were PFC negative, whereas up to 20% of immune cells in the ECM were PFC negative. The highest proportion of infiltrating cells consisted of neutrophils followed by macrophages in the peri-infarct area, as well as the ECM hydrogel. Only a small proportion (<2%) were lymphocytes in the peri-infarct area. No lymphocytes were present in the ECM hydrogel.
Conclusion: PFC nanoemulsion detection using 19F MRI provides a unique tool to characterize the spatio-temporal dynamics of labelled-immune cell infiltration into brain tissue and bioscaffolds. This approach will be essential to improve our understanding of how the immune system mediates bioscaffold-induced tissue regeneration.
Acknowledgements
No acknowledgement found.References
[1.] Ghuman H, Mauney C, Donnelly J, Massensini AR, Badylak SF, Modo M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018;80:66-84. Epub 2018/09/21. doi: 10.1016/j.actbio.2018.09.020. PubMed PMID: 30232030; PMCID: PMC6217851.
[2.] Ghuman H, Hitchens TK, Modo M. A systematic optimization of (19)F MR image acquisition to detect macrophage invasion into an ECM hydrogel implanted in the stroke-damaged brain. Neuroimage. 2019;202:116090. Epub 2019/08/14. doi: 10.1016/j.neuroimage.2019.116090. PubMed PMID: 31408717; PMCID: PMC6819274.