1012
MultI-Compartment Model based Parametric Mapping of the Heart with a Single MOLLI Acquisition (MIC-MOLLI)
Jing Liu1, Karen Ordovas2, Yan Wang1, Janine Lupo1, Duan Xu1, Yoojin Lee1, Roselle Abraham1, and David Saloner1
1University of California San Francisco, San Francisco, CA, United States, 2University of Washington, Seattle, WA, United States
1University of California San Francisco, San Francisco, CA, United States, 2University of Washington, Seattle, WA, United States
Synopsis
In this study, we developed a multi-compartment model based method for deriving multi-parametric mapping of the heart from the MOLLI T1 mapping acquisition. The preliminary results on patients with hypertrophic cardiomyopathy demonstrated the feasibility of deriving 9 quantitative parametric maps from a single scan and showed its potential of providing a comprehensive assessment of the myocardial tissue composition.
Introduction
MRI techniques with contrast enhancement or quantitative parametric mapping allow myocardial tissue characterization, which have been broadly used for discovering the underlying tissue composition that reflects disease progression such as for assessing myocardial fibrosis. We developed a multi-compartment model based multi-parametric mapping technique from MOLLI acquisition (so called MIC-MOLLI) to quantitatively characterize tissue composition, providing the distribution of multiple myocardial water pools (free water, bound water and macromolecular proton), which are expected to be associated with loss of myocytes and increase of myocardial collagen deposition in diseased heart.Methods
Magnetization Transfer (MT) effect has been explored for assessing myocardial fibrosis using multiple off-resonance preparation pulses [1] or combining MT, T1 and proton-density contrast weightings [2]. Both methods attempted to quantifying myocardial macromolecular proton fraction, which is expected to reflect the changes of collagen deposition associated with myocardial fibrosis. Our approach explores the widely used T1 mapping MOLLI sequence. Although MOLLI sequence with bSSFP acquisition has been used for measuring myocardial T1 values, the data for fitting the T1 maps has inherent information of relaxation related to T2 decay and magnetization transfer in the tissue besides to the T1 relaxation, which could be explored for tracking the underlying tissue composition. Three-compartment model (free water, bound water and macromolecular proton, Figure 1 left column) was built based on the Bloch-McConnel equations for generating instant signal evolution during MOLLI acquisition, given scan parameters and pre-defined ranges of tissue parametric parameters, forming a dictionary of signal evolution curves. An iterative matching algorithm was developed to derive a series of quantitative parameters, including T1 and T2 mapping of free water and bound water respectively, and the fractions for free water, bound water and macromolecular proton pools, respectively. Given limited data points using MOLLI, 11 for 3(3)3(3)5 or 8 for 5(3)3 patterns, it is challenging to derive reliable multiple parameters using the three-compartment model. In this study, we simplified the model to two two-compartment models (as shown in Figure 1 middle and right columns), which allow us to derive the targeted list of the parameters with feasible dictionary search. We applied our method on the MOLLI data using 5(3)3 pattern acquired on 3T MR scanner (GE Healthcare, Milwaukee) from 9 Hypertrophic Cardiomyopathy (HCM) patients with replacement fibrosis identified in LGE images. The maps derived from the multi-compartment models were compared to those using the gold standard methods - extracellular volume (ECV) mapping (from pre- and post-contrast T1 mapping) as well as late Gadolinium enhancement (LGE), respectively. ECV maps were calculated where hematocrit (HCT) was estimated by fitting through the pre-contrast T1 values of the blood [3]. Regions of normal myocardium, replacement fibrosis and gray zone were segmented on LGE images using full width at half maximum (FWHM) method. The regions of the myocardium in the images acquired with MOLLI (pre-contrast) were automatically segmented and registered to those acquired in post-contrast T1 and LGE images.Methods
Figure 2 shows the LGE image (first column), the conventional pre- and post-contrast T1 and ECV maps (second column), as well as 9 parametric maps derived using MIC-MOLLI method. Figure 3 shows the segmentation of normal myocardium, scar and gray zone based on LGE images (same case in Figure 2). The values of the parametric mapping in those three regions were plotted Figure 4, averaged among the 9 cases. Multiple parameters derived from MIC-MOLLI show increase or decrease measurements in fibrosis again normal myocardium. Overall, T1 and T2 values increase in fibrosis, macromolecular proton and bound water fractions decreases, and free water fraction increases. Correlations between some of parameters derived from MIC-MOLLI and ECV are shown in Figure 5. The T1 with two-compartment model A and Bound water fraction with two-compartment model B provide stronger correlations than the conventional T1 (r=0.77 and -0.68 vs r=0.59).Discussion
Similar to the results reported in [1][2], the macromolecular proton fraction in fibrosis decreases compared to the normal myocardium. Although fibrosis is associated with increased deposition of collagen (connective tissue protein), the loss of myocytes that have rich amount of proteins may play a more significant role in terms of the measurement of macromolecular proton fraction. The current methods do not specify the sources of macromolecules.Conclusion
We have demonstrated multi-compartment model based cardiac multi-parametric mapping from non-contrast enhanced MOLLI acquisition for assessing myocardial fibrosis on patients with hypertrophic cardiomyopathy. This approach has the potential of providing comprehensive and reliable evaluation of the myocardial fibrosis.Acknowledgements
No acknowledgement found.References
1. K. Lopez, et. al. Magn Reson Med. 2021 Apr;85(4):2069-2083. doi: 10.1002/mrm.28577.
2. A. V. Naumova, et. al. ISMRM 2020, #2073.
3. Z. Zhou, et. al. Quant Imaging Med Surg 2021 Feb;11(2):510-520. doi: 10.21037/qims-20-501.
Figures
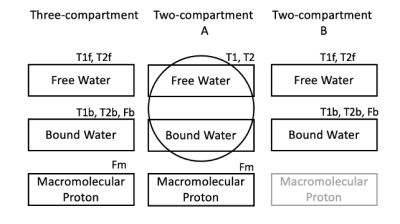
Figure 1. Three-compartment model is based on the Bloch-McConnel equations that describe the exchange process between multiple compartments (including MT effect). Simplified two-compartment models (A&B) were used to derive multi-parametric mapping from a single MOLLI acquisition.

Figure 2 LGE image, conventional pre- and post-contrast T1 and ECV maps (second column), and 9 parametric maps derived using MIC-MOLLI method.

Figure 3. Segmentation of normal myocardium, scar and gray zone from the case in Figure 2.
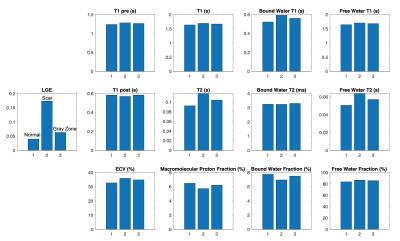
Figure 4. Averaged values of the parametric mapping in normal myocardium, scar and gray zone, respectively (three bars), from 9 HCM cases. (LGE image signals were normalized to be between 0 and 1).
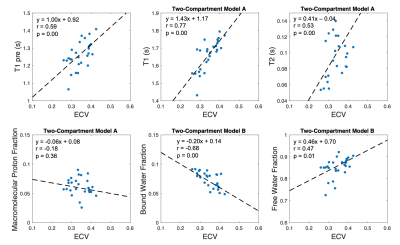
Figure 5. Correlations between ECV and the conventional T1 values, as well as T1, T2, fractions of macromolecular proton, bound water and free water derived with MIC-MOLLI method, were calculated based on the measurements in the three segments of the 9 cases. T1 values with the two-compartment model A and the fraction of bound water fraction have strong correlations (r=0.77 and r= -0.68) with ECV.
DOI: https://doi.org/10.58530/2022/1012