1009
Dynamic UTE molecular MR imaging targeting pulmonary fibrogenesis in a model of left ventricular dysfunction1Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Radiology, Institute for Innovation in Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Medicine, Division of Cardiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 4Medicine, Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 5Pulmonary, Critical Care and Sleep Medicine, Tufts Medical Center, Boston, MA, United States
Synopsis
Group 2 pulmonary hypertension is a complication of chronic left ventricular dysfunction. During the early stages of the disease activity there is an accumulation of extracellular matrix molecules and an increase in allysine content. Molecular MR imaging with an allysine-targeted fibrogenesis probe shows increased lung-to-muscle ΔCNR within the lungs of transverse aortic constriction (TAC)-induced left ventricular dysfunction animal models compared to control animals, which corresponded to increased lung and right ventricle weight, and elevation of fibrogenesis biomarkers (hydroxyproline and allysine).
Purpose
Left ventricular dysfunction promotes Group 2 pulmonary hypertension (PH) and right ventricular failure1,2, which produces pulmonary congestion, fibrosis, and increased lung weight3. Current diagnostic approaches include echocardiogram and pulmonary function tests to assess pulmonary arterial pressure and right heart catheterization to confirm diagnosis of PH. However, there is a lack of noninvasive methods available to detect early onset of disease and assess disease progression. Here, we use molecular MR imaging to detect pulmonary fibrogenesis (active fibrosis) by targeting allysine residues formed on matrix proteins due to lysyl oxidase (LOX) enzymatic activity that is upregulated during disease activity4,5. The objective of this study was to evaluate and validate the presence of pulmonary fibrogenesis and PH in an animal model of chronic left ventricular dysfunction using molecular MRI with an allysine-targeting probe. The disease model was characterized by assessing lung and RV weights and investigating the association of molecular MRI findings with biomarkers of fibrosis (hydroxyproline) and fibrogenesis (allysine).Methods
Left ventricular dysfunction with PH was induced by left thoracotomy transverse aortic constriction surgery (TAC)3,6,7 in 6-month-old senescence-accelerated mouse resistant stain (SAMR1, female n=2, male n=3). The transverse aorta was encircled with 7-0 nylon suture and tied tightly around a pre-sterilized, blunt-end of a 27-gauge needle. After securing the knot, the needle was removed, and aortic flow resumed. Sham surgery was performed in 6-month SAMR1 (female n=1, male=3) control mice. 3-weeks post-surgery, mice were anesthetized with isoflurane (1.5%) and imaged at a 4.7 Tesla Bruker MRI scanner. Ultrashort Echo Time (UTE) MR imaging (TR/TE=4/0.01225 msec, NEX=1, FA=16 degrees, voxel size=0.25x0.25x0.25 mm3, BW=781 Hz/pixel) was acquired prior to and up to 40 minutes post intravenous administration of 100 µmol/kg Gd-1,4, a dual binding probe that targets fibrogenesis through nucleophilic reaction with allysine8. ROI analysis of the lungs was performed in AMIDE pre- and post-injection. CNR was calculated at each timepoint by (SIlung – SImuscle)/SDphantom, ΔCNR was calculated as CNRpost-injection – CNRpre-injection and AUC was computed using trapezoidal integration. After MRI, mice were euthanized 60 minutes post-injection, the right lung (RL), left (lung) and heart tissue (right ventricle (RV), left ventricle (LV), septum) were collected, weighed, and analyzed for hydroxyproline (Hyp) and allysine content4. A subset of cardiac tissue was collected fixed and stained with Sirius Red. Student’s t-test comparison was performed between TAC and control mice (results are reported as mean±SD, significance if p<0.05).Results
Transverse aortic constriction (TAC) for a duration of 3 weeks led to active pulmonary fibrosis (fibrogenesis), pulmonary hypertension, and myocardial tissue remodeling as assessed by MRI, tissue weights, Hyp, allysine, and histology. Representative UTE MR images acquired before and after injection of Gd-1,4 are shown in Figure 1A. Compared to control mice, lung to muscle ΔCNR remained elevated post-injection and the AUC of ΔCNR versus post-injection time was significantly increased (RL: AUCTAC=49±13, AUCCtrl=16±3, P=0.01; LL: AUCTAC=54±13, AUCCtrl=11±6, P=0.004, Figure 1B,C) indicating binding of the Gd-1,4 allysine-targeted fibrogenesis probe and the presence of pulmonary fibrogenesis. Ratio of lung weight to body weight was significantly increased in the TAC mice compared to control mice (RL: 7.7±2.5 versus 2.5±0.32 mg/g, P<0.001; LL: 3.4±1.2 versus 1.3±0.12 mg/g, P=0.019, Figure 2A,B). Additionally, the ratio of right ventricle weight to body weight (marker of pulmonary hypertension3) was significantly increased in the TAC mice (1.3±0.29 versus 0.70±0.14 mg/g, P<0.001, Figure 2C). Hyp and allysine were elevated in the lungs of TAC mice (RL: HypTAC=198±93, HypCtrl=114±8 µg/lung, P=0.25; LL: allysineTAC=4.2±2.0, allysineCtrl=1.6±1.5 nmol/lung, P=0.15, Figure 2D,E) indicating pulmonary fibrosis and fibrogenesis, respectively. TAC mice demonstrated elevated Hyp and allysine content in myocardial tissue indicating the presence of fibrosis and tissue remodeling (LV: HypTAC=811±166, HypCtrl=446±141 µg/g, P=0.04; Septum: allysineTAC=8.7±4.9, allysineCtrl=5.6±4.0 nmol/g, P=0.38, Figure 3A,B). Histology showed positive staining of fibrosis in the myocardium (Figure 3C).Discussion and Conclusion
The major finding of the present study was the significant elevation of lung to muscle ΔCNR post-injection of the Gd-1,4 allysine-targeted probe, which signifies the presence of active pulmonary fibrosis and PH in a mouse model of chronic transverse aortic constriction (TAC)-induced left ventricular dysfunction. The pressure overload induced by TAC is known to cause muscularized pulmonary arteries, lung fibrosis, myofibroblast proliferation and immune response3. Previous molecular imaging studies with Gd-1,4 in an animal model of liver fibrosis showed this probe to be sensitive in detecting early-stage disease8. The sensitivity of Gd-1,4 fibrogenesis imaging of PH correlated with the detection of elevated fibrosis markers (Hyp and allysine) and significant increases in lung and right ventricle weight (marker of PH3). The data showed strong signal in a limited number of mice but needs to be confirmed with increased sample size. PH is secondary to left ventricular dysfunction9. Adverse myocardial remodeling was detected through elevated Hyp and allysine measurements and positive Sirius Red fibrosis staining. Overall, fibrogenesis imaging shows potential for detecting early-stage PH. Future work would include a longitudinal study to assess disease progression and therapeutic response in mouse models of ageing-associated PH and left ventricular dysfunction.Acknowledgements
We gratefully acknowledge funding support from the National Institute on Aging (R01AG064064 to Penumatsa), the National Heart Lung and Blood Institute (R33HL154125 to Caravan), and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK121789 to Caravan).References
1. Lai, Y.C., Wang, L. & Gladwin, M.T. Insights into the pulmonary vascular complications of heart failure with preserved ejection fraction. J Physiol. 2019;597:1143-1156. doi: 10.1113/JP275858.
2. Haddad, F., Doyle, R., Murphy, D.J., et al. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717-1731. doi: 10.1161/CIRCULATIONAHA.107.653584.
3. Chen, Y., Guo, H., Xu, D., et al. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170-1178. doi: 10.1161/HYPERTENSIONAHA.111.186072.
4. Waghorn, P.A., Oliveira, B.L., Jones, C.M., et al. High sensitivity HPLC method for determination of the allysine concentration in tissue by use of a naphthol derivative. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1064:7-13. doi: 10.1016/j.jchromb.2017.08.032.
5. Chen, H.H., Waghorn, P.A., Wei, L., et al. Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI Insight. 2017;2:doi: 10.1172/jci.insight.91506.
6. Reed, A.L., Tanaka, A., Sorescu, D., et al. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol. 2011;301:H824-831. doi: 10.1152/ajpheart.00407.2010.
7. Penumatsa, K.C., Falcao-Pires, I., Leite, S., et al. Increased Transglutaminase 2 Expression and Activity in Rodent Models of Obesity/Metabolic Syndrome and Aging. Front Physiol. 2020;11:560019. doi: 10.3389/fphys.2020.560019.
8. Zhou, I.Y., Ning, Y., Rotile, N.J., et al. Quantitative MR imaging of liver fibrogenesis in nonalcoholic steatohepatitis (NASH). in World Molecular Imaging Congress (Virtual, 2021).
9. Dai, Z.K., Tan, M.S., Chai, C.Y., et al. Upregulation of endothelial nitric oxide synthase and endothelin-1 in pulmonary hypertension secondary to heart failure in aorta-banded rats. Pediatr Pulmonol. 2004;37:249-256. doi: 10.1002/ppul.10413.
Figures

Figure 1: Transverse aortic constriction (TAC) leads to pulmonary fibrogenesis. A) Pre-injection UTE imaging was performed in control and TAC mice followed by injection of Gd-1,4 and dynamic UTE imaging. B) RL and C) LL signal intensity remained elevated post-injection indicating the presence of pulmonary fibrogenesis and the AUC of ΔCNR versus post-injection time were significantly elevated. *P<0.05 and **P<0.001.
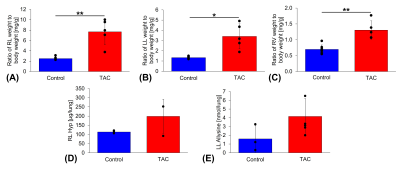
Figure 2: Transverse aortic constriction (TAC) causes pulmonary and cardiac tissue remodeling. Ratio of A) right lung (RL), B) left lung (LL), and C) right ventricle (RV) weight to body weight were significantly increased in TAC mice compared to control mice. D) RL hydroxyproline (Hyp, marker of fibrosis, [Hyp] in micrograms per gram of tissue) and E) LL allysine (marker of fibrogenesis, [allysine] in nmol per gram of tissue) content were elevated in TAC mice. *P<0.05 and **P<0.001.
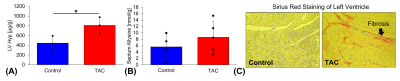
Figure 3: Myocardial fibrosis was detected in transverse aortic constriction (TAC)-induced left ventricular dysfunction model. A) Hydroxyproline content (Hyp, marker of fibrosis, [Hyp] in micrograms per gram of tissue) measured from the entire left ventricle (LV) and B) allysine content (marker of fibrogenesis, [allysine] in nmol per gram of tissue) measured in from the entire septum were elevated in TAC mice. C) Sirius Red histochemistry showed positive staining of myocardial fibrosis in a cross section view of the left ventricle. *P<0.05 and **P<0.001.