1003
Individualized arterial spin labeling background suppression by rapid T1 mapping during acquisition1Department of Diagnostic and Interventional Neuroradiology, University Hospital Hamburg-Eppendorf, Hamburg, Germany
Synopsis
This work presents a method of rapidly acquiring a T1 map to optimize background suppression in Arterial Spin Labeling to improve the signal-to-background contrast.
Introduction
Arterial Spin Labeling (ASL) is a known technique for the non-invasive evaluation of cerebral blood flow (CBF) [1]. The technique produces relative perfusion values by subtracting the label and control image that are obtained in an interleaved fashion. To obtain maximum signal-to-background contrast in the final (perfusion) image, background suppression pulses are commonly used [2]. These pulses aim to cancel the signal of static tissue (gray and white matter) while blood difference signal should remain maximum. The timing of these pulses has commonly been obtained by Bloch simulations of the signal course of tissues with values taken from the literature. This study aims to improve background suppression pulse timing by incorporating rapid T1 mapping of static tissue into the ASL sequence, thereby improving the final images.Materials and Methods
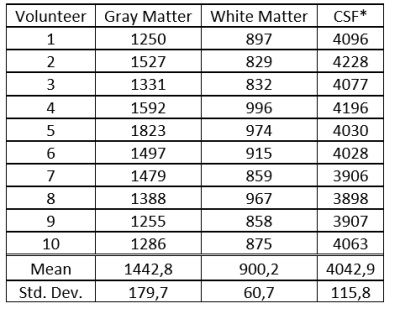
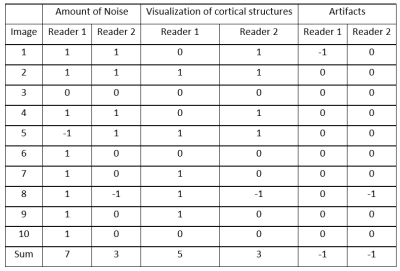
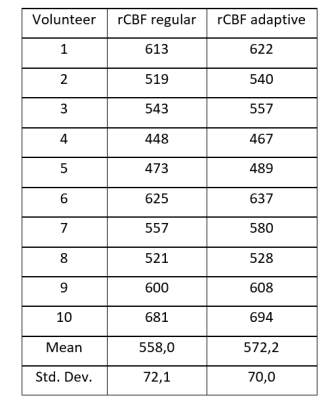
The test collective consists of 10 healthy volunteers (4 women, 6 men, mean age 26.8 years). All subjects underwent scanning on a 3T MAGNETOM Prisma scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel head coil. The study was approved by the local ethical committee, volunteers gave written informed consent. Scan parameters included: 2000ms labeling duration and 1700ms post labeling delay, 3D GraSE readout with 3.6x3.6x4mm³ resolution, TR/TE: 400/12.06ms. The T1 maps were obtained by the variable flip angle method using qMRLAB [3,4]. Thus, one acquisition was performed with a single label/control pair with a flip angle (FA) of 9° for label and 20° for control and one was acquired with the FAs switched (i.e. 20° FA for control and 9° FA for label). Acquisition of such a label/control pair took 1:12 minutes. The “conventional” ASL acquisition was performed using 20° FA with 4 label/control pairs. Segmentation of Gray and White matter and CSF was performed using SPM12 (The Wellcome Centre for Human Neuroimaging, London, UK). Based on the segmented areas, a mean value of T1 was calculated and used for optimum background suppression timing. Differences in relative (rCBF) were assessed using the Wilcoxon-Signed Ranked test. Visual rating was performed by two independent readers on a three-point grading scale in three categories.Results and Discussion
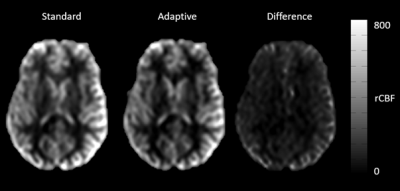
Using ASL for rapid T1 mapping provided values comparable to the literature (Table 1). Adapting the background suppression pulses to the individual values show higher signal to background ratio as compared to a fixed (standard) setting of the background suppression pulses (Figure 1). This was confirmed by the readers except for artifacts, which are shown to be the same in most cases, but worse in two cases (Table 2). Differences in rCBF can be seen in table 3. These results show a p-value of 0.05. There was no difference in results whether the label or control condition was acquired using 9° or 20° FA respectively for mapping (Figure 2).Conclusion
Individualization of the background suppression pulse timing can improve the signal to background ratio in ASL subtraction images, thus improving the visual impression. It needs to be proven whether the scan time extension caused by adding one more condition that cannot be used for creating perfusion images justifies the results obtained in a clinical setting.Acknowledgements
No acknowledgement found.References
[1] Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102-116. doi:10.1002/mrm.25197
[2] Garcia DM, Duhamel G, Alsop DC. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med. 2005;54(2):366-372. doi:10.1002/mrm.20556
[3] Cabana JF et. al. Quantitative magnetization transfer imaging made easy with qMTLab: Software for data simulation, analysis, and visualization. Concepts Magn Reson. 2016;44A(5):263-277
[4] Fram EK, Herfkens RJ, Johnson GA, et al. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging. 1987;5(3):201-208. doi:10.1016/0730-725x(87)90021-x
Figures