0999
Temporal resolution impacts in-vivo human brain dynamic functional MRI connectivity
Francesca Saviola1, Stefano Tambalo1, Dimitri Van De Ville2,3, and Jorge Jovicich1
1University of Trento, Center for Mind/Brain Sciences, Rovereto, Italy, 2Institute of Bioengineering, Center for Neuroprosthetics, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Radiology and Medical Informatics, University of Geneva (UNIGE), Geneva, Switzerland
1University of Trento, Center for Mind/Brain Sciences, Rovereto, Italy, 2Institute of Bioengineering, Center for Neuroprosthetics, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Radiology and Medical Informatics, University of Geneva (UNIGE), Geneva, Switzerland
Synopsis
The advent of fast fMRI acquisition techniques has enabled whole-brain acquisitions with sub-second temporal resolution enriching the information available throughout the time course. However, there is no consensus about how the application of frame-wise analysis, performed to better understand functional brain fluctuations, could benefit from high temporal resolution fMRI. In this work, we demonstrate the potential need to: (i) gain a finer understanding of signal and noise signatures peculiar to fast acquisition and (ii) extend the models to better estimate dynamic functional connectivity in rapidly sampled fMRI time series.
Introduction
In recent years, two separate methodological developments have opened new avenues to study human brain function non-invasively. On the one hand, MRI technological progress has enabled fast functional MRI (fMRI, full brain volume under 1 s) in clinical scanners[1,2]. On the other hand, novel analytical methods have been proposed to study the dynamics of functional brain connectivity, and these methods are already being used to search for novel disease biomarkers[3-9]. However, these two separate developments have not been fully integrated. Currently, there is poor knowledge about how functional dynamic metrics depend on basic acquisition fMRI variables, such as temporal resolution. This makes the comparison of studies in the literature challenging, and potentially leaves open the question about how optimal some protocols may be to study clinical populations.Materials and Methods
A total of 20 healthy controls (HC,10 females; age 24±3 years) participated in this study. Data were acquired with a 3T Siemens Prisma MRI scanner equipped with a 64 channels receive-only head coil. Structural T1-weighted MPRAGE (TR/TE=2.31s/3.48ms, 1mm-isotropic voxels) and three resting-state fMRI (rs-fMRI) runs with varying TR (1s, 2s and 3s) with the same TE=28 ms, 3mm-isotropic voxels, SMS=6, no parallel imaging and TA=7.5 min were used to test the effects of TR on temporal-varying network estimations. All sessions were pre-processed using common pre-processing steps including: (1) slice timing and head motion correction; (2) co-registration of the T1-weighted image to the rs-fMRI time-series; (3) T1-weighted image tissue segmentation; (4) rs-fMRI temporal detrending and median filtering; (5) regression from the rs-fMRI time-series of the 6 head motion parameters, white matter, and cerebrospinal fluid signals; (7) normalization to standard MNI template space; (8) spatial smoothing with 6 mm FWHM Gaussian kernel size. Dynamic functional connectivity analysis (dFC) was computed through innovation-driven co-activation patterns (iCAPs) in Matlab (https://c4science.ch/source/iCAPs/). This allowed estimation of: (i) total duration percentage of each functional network (iCAP); (ii) coupling and anti-coupling measures of two iCAPs. Duration and coupling measures between rs-fMRI protocols were compared using a paired t-test between pairs of acquisitions (TR 3s versus TR 2s, TR 3s versus TR 1s, and TR 2s versus TR 1s) and corrected for multiple comparisons using false-discovery rate (FDR).Results
Consensus clustering on the longest TR (TR=3s, Figure 1) determined k=11 as the best performing k-mean value, resulting in 11 iCAPs reminiscent of well-known rs-fMRI networks[10]. From a spatial point of view, for both TR 3s and TR 2s runs, all 11 iCAPs were highly correlated between them and with well-known functional networks (Fig. 1). On the other hand, the TR 1s gave only 4/11 iCAPs: namely Auditory (AUD), Visual (VIS), anterior Salience (aSN), Frontal (FRON) and Default mode (DMN) networks. The total duration percentage of iCAPs did not statistically differ between the TR 3s and TR 2s runs, suggesting the high robustness of the networks regardless of the change in the resting cognitive state of the participants. However, the TR 1s run, with only 5 relevant cognitive iCAPs present in all frames, was characterized by increased total duration on each iCAPs relative to TR 3s (AUD: pFDR<0.01, t=-5.2; VIS: pFDR<0.01, t=-4.6; aSN: pFDR<0.01, t=-6.8; FRON: pFDR<0.01, t=-4.3; DMN: pFDR<0.01, t=-6.3) and TR 2 s (besides the Frontal network, AUD: pFDR<0.01, t=-4.1; VIS: pFDR<0.01, t=-7.4; aSN: pFDR<0.01, t=-6.6; DMN: pFDR<0.01, t=-7.6). Indeed, from a spatial perspective, the remaining 7 iCAPs of the TR 1s session appear to reflect sparse noisy activation, with temporal properties frequently close to zero throughout the time course for all subjects.Discussion and Conclusions
Our results suggest that studying in-vivo brain dynamic functional connectivity with iCAPs is robust for TRs fMRI acquisitions in the range ~ 2-3 s. However, faster sampling rates (~ 1s) with our spatial resolution (27 mm3 voxels) seem to disrupt both the spatial reconstruction of dynamic brain states and their temporal properties regardless of its temporal resolution richness. Previous studies demonstrated the possibility to reconstruct iCAPs at high temporal resolution (~ 1.1 s) with larger voxels (78 mm3)[11]. The higher spatial resolution of our protocol, with its reduced signal-to-noise ratio (SNR) effects, may help explain the challenges in characterizing dynamic properties in the time series. Our results suggest the potential need to adjust the iCAPs framework in order to enable a robust estimation of brain dynamic properties for high resolution fast fMRI acquisition. Potential considerations include: (i) the lower SNR of fast fMRI acquisition[2], (ii) a potential change in the hemodynamic-response function used in iCAPs deconvolution pipeline[12], (iii) the sampling of higher frequencies potentially affecting the total activation process[13].Acknowledgements
ISMRM Exchange Award 2021-2022 “Investigating in-vivo human brain dynamic connectivity with fast fMRI”, Dipartimento di Eccellenza project 2018-2022 (Italian Ministry of Education, University and Research).References
- Lewis, L. D., Setsompop, K., Rosen, B. R., & Polimeni, J. R. (2016). Fast fMRI can detect oscillatory neural activity in humans. Proceedings of the national academy of sciences, 113(43), E6679-E6685.
- Chen, J. E., Polimeni, J. R., Bollmann, S., & Glover, G. H. (2019). On the analysis of rapidly sampled fMRI data. Neuroimage, 188, 807-820.
- Liégeois, R., Li, J., Kong, R., Orban, C., Van De Ville, D., Ge, T., ... & Yeo, B. T. (2019). Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nature communications, 10(1), 1-9.
- Vidaurre, D., Smith, S. M., & Woolrich, M. W. (2017). Brain network dynamics are hierarchically organized in time. Proceedings of the National Academy of Sciences, 114(48), 12827-12832.
- Jin, C., Jia, H., Lanka, P., Rangaprakash, D., Li, L., Liu, T., ... & Deshpande, G. (2017). Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Human brain mapping, 38(9), 4479-4496.
- Rashid, B., Arbabshirani, M. R., Damaraju, E., Cetin, M. S., Miller, R., Pearlson, G. D., & Calhoun, V. D. (2016). Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage, 134, 645-657.
- Zöller, D., Sandini, C., Karahanoğlu, F. I., Padula, M. C., Schaer, M., Eliez, S., & Van De Ville, D. (2019). Large-scale brain network dynamics provide a measure of psychosis and anxiety in 22q11. 2 deletion syndrome. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(10), 881-892.
- Bommarito, G., Tarun, A., Farouj, Y., Preti, M. G., Petracca, M., Droby, A., ... & Van De Ville, D. (2021). Altered anterior default mode network dynamics in progressive multiple sclerosis. Multiple Sclerosis Journal, 13524585211018116.
- Bolton, T. A., Wotruba, D., Buechler, R., Theodoridou, A., Michels, L., Kollias, S., ... & Van De Ville, D. (2020). Triple network model dynamically revisited: lower salience network state switching in pre-psychosis. Frontiers in physiology, 11, 66.
- Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., & Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex, 22(1), 158-165.
- Karahanoğlu, F. I., & Van De Ville, D. (2015). Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nature communications, 6(1), 1-10.
- Polimeni, J. R., & Lewis, L. D. (2021). Imaging faster neural dynamics with fast fMRI: a need for updated models of the hemodynamic response. Progress in neurobiology, 102174.
- Karahanoğlu, F. I., Caballero-Gaudes, C., Lazeyras, F., & Van De Ville, D. (2013). Total activation: fMRI deconvolution through spatio-temporal regularization. Neuroimage, 73, 121-134.
Figures
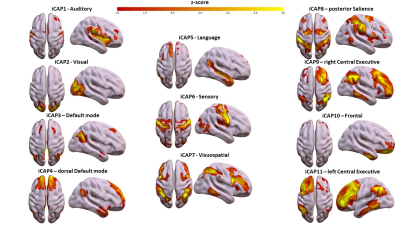
Spatial patterns of the 11 innovation-driven coactivation patterns (iCAPs) were retrieved from a group of healthy adult subjects (N=20) in the first session (TR=3 s).
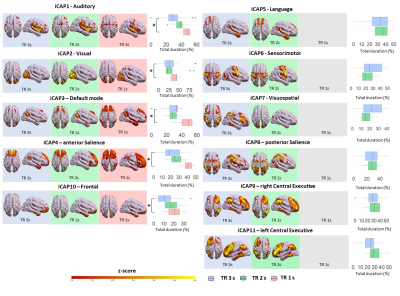
Spatial patterns of the 11 innovation-driven coactivation patterns (iCAPs) were retrieved from all subjects in all sessions (blue: TR=3 s, green: TR=2s; red: TR=1s). For each iCAPs, horizontal boxplots show the innovation frames per session as a percentage of the total scanning time, highlighting the differences between TR 3s and TR 1s runs (*=p<0.05FDR).
DOI: https://doi.org/10.58530/2022/0999