0984
Diffusion dispersion and microscopic fractional anisotropy reveal acute sensitivity to mild traumatic brain injury in a mouse model1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Anatomy and Cell Biology, Western University, London, ON, Canada
Synopsis
Imaging markers of mild to moderate concussion are notoriously difficult to detect in vivo. Advanced diffusion MRI (dMRI) techniques have shown increased sensitivity and specificity to microstructural changes in various disease and injury models. Oscillating gradient spin-echo (OGSE) dMRI is sensitive to structural disorder and microscopic anisotropy (µA) dMRI is sensitive to water diffusion anisotropy independent of neuron fiber orientation. In this work, we demonstrate that both microscopic fractional anisotropy and diffusion dispersion show acute sensitivity to concussion, while traditional diffusion MRI markers do not.
Introduction
Current neuroimaging techniques lack the specificity required to reliably detect signs of mild traumatic brain injury (mTBI) [1]. Microstructure imaging with advanced diffusion MRI (dMRI) techniques have shown increased sensitivity and specificity to microstructural changes in various disease and injury models. Oscillating gradient spin echo (OGSE) dMRI [2] and microscopic anisotropy (µA) dMRI [3] may provide additional insight by increasing sensitivity to smaller spatial scales and disentangling fiber orientation dispersion from true microstructural changes, respectively. Here, we evaluate mean diffusivity difference (ΔMD: a measure of the diffusion dispersion rate, which characterizes MD dependence on OGSE frequency), microscopic fractional anisotropy, and traditional dMRI metrics longitudinally in sham and concussed mice.Methods
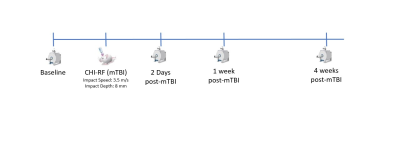
The sham and concussed cohort each consisted of six female C57Bl/6 mice, aged 10-12 weeks at the start of the study. Longitudinal imaging was performed on the sham and concussed cohort at baseline, 2 days post-mTBI, 1-week post-mTBI, and 4 weeks post-mTBI (Fig. 1). The mTBI model used here is the CHI-RF (cortical head injury with rotational force) model, which is designed to elicit a mild concussion as a result of both linear and rotational forces, by matching the stretch/strain in the rodent brain during mTBI to that experienced by the human brain, as measured in athletes [4].Imaging was performed at 9.4T with a 1 T/m gradient insert using single-shot EPI with an in-plane resolution of 0.175mm x 0.2mm, 0.5mm slice thickness, and a total scan time of 2 hours. The OGSE sequence was implemented with b=800s/mm2, TE=37ms, 10 directions and OGSE frequencies of 0, 50, 100, 145, and 190 Hz. The µA sequence was implemented using a single diffusion encoding (SDE) scheme with linear and spherical tensor encodings at b=2000s/mm2 (30 directions) and b=1000s/mm2 (12 directions) [5]. Post processing included PCA denoising [6] and eddy current correction with FSL [7]. Parameters were measured in the corpus callosum (CC) and prefrontal cortex (PFC). For each metric, paired t-tests were performed between each timepoint post-mTBI and the baseline for each cohort.
Results
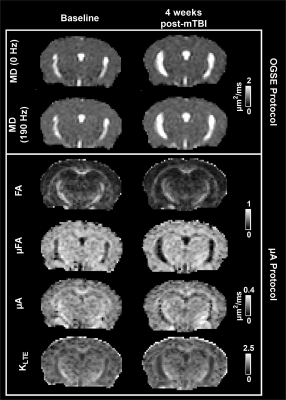
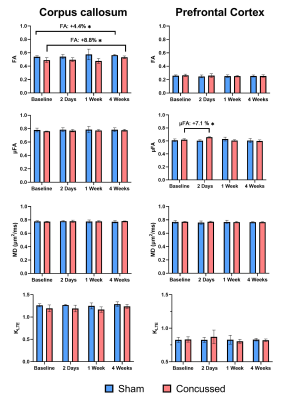
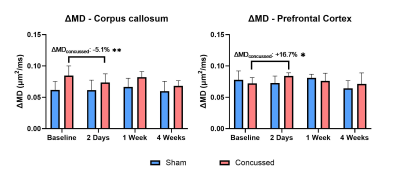
Parameter maps at baseline and 4 weeks post-mTBI, in one concussed mouse, are shown in Fig. 2. In the PFC, a 7.1 % increase in µFA (Fig. 3) and a 16.7 % increase in ΔMD (Fig. 4) was found 2 days post-mTBI, compared to baseline. In the CC, a 5.1 % decrease in ΔMD was found 2 days post-mTBI (Fig. 4). No significant changes were found in the traditional dMRI metrics, except an increase in FA in the CC for both sham and concussed cohorts.Discussion
While imaging markers of mild concussion are challenging to detect in vivo, the first application of OGSE and µA dMRI in a mild concussion model shows acute sensitivity to concussion. In the PFC, the increase in ΔMD is consistent with neurite beading [8,9] and with preliminary results in the PFC in one mouse 2 days post-mTBI [10]. This is accompanied by an increase in µFA 2 days post-mTBI. However, simulation has predicted a µFA decrease with beading [11]. Simulations have also shown a µFA increase with increasing intracellular compartment volume fraction, which may indicate the presence of cytotoxic edema here. Glial cell processes, such as those present in astrogliosis may also result in highly anisotropic water diffusion. In a previous rodent TBI model (resulting in a more traumatic injury than the mTBI model used in this work), an increase in KLTE in the cortex was associated with increased reactive astrogliosis [12]. This may explain the non-significant increase in KLTE in the PFC at 2 days post-mTBI in our milder model, accompanied by a significant increase in µFA. Although most studies have reported a reduction in µFA in various pathologies [13–15], recently, elevated µFA in acute stroke has been hypothesized to reflect increased trapped water in swollen axons [16].In contrast to the PFC, a decrease in ΔMD is found in the CC at 2 days post-mTBI. There is a trend of increasing ΔMD between 2 days and 1-week post-mTBI, although not significant with a sample size of 6 mice. The decrease and subsequent increase of ΔMD may provide new insight into the interplay of beading and inflammation during concussion recovery. From a preliminary study involving a mild and severe TBI model, we hypothesize that beading and inflammation may affect OGSE contrast in opposing ways [10]. The effect of inflammation on OGSE contrast remains to be explored.
The only change in traditional dMRI metrics is an increase in FA in the CC, at the 4-week timepoint, for both sham and concussed cohorts. This increase may be related to brain maturation, as myelination continues to increase in mice between three and six months [17], and the mice used in this study were 10-12 weeks old at baseline. This merits investigation into MRI changes in healthy mice over time, which remains largely unexplored. Although a decrease in MD has been reported in the acute stage in various rodent TBI models [18–20], no changes in MD are found in this mild concussion model.
In conclusion, we demonstrate that both µFA and diffusion dispersion show acute sensitivity to concussion, while traditional dMRI markers do not.
Acknowledgements
Natural Sciences and Engineering Research Council of Canada (NSERC)
Ontario Graduate Scholarship (OGS)
Canada First Research Excellence Fund to BrainsCAN
New Frontiers in Research Fund (NFRF)
References
1. Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, et al. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage Clin. 2014;4:283–94. Available from: http://dx.doi.org/10.1016/j.nicl.2013.12.009
2. Baron CA, Beaulieu C. Oscillating gradient spin-echo (OGSE) diffusion tensor imaging of the human brain. Magn Reson Med. 2014;72(3):726–36.
3. Lasič S, Szczepankiewicz F, Eriksson S, Nilsson M, Topgaard D. Microanisotropy imaging: Quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Front Phys. 2014;2(February):1–14.
4. Bodnar CN, Roberts KN, Higgins EK, Bachstetter AD. A Systematic Review of Closed Head Injury Models of Mild Traumatic Brain Injury in Mice and Rats. J Neurotrauma. 2019;36(11):1683–706.
5. Arezza NJJ, Tse DHY, Baron CA. Rapid microscopic fractional anisotropy imaging via an optimized linear regression formulation. Magn Reson Imaging. 2021;80(April):132–43. Available from: https://doi.org/10.1016/j.mri.2021.04.015
6. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. Available from: http://dx.doi.org/10.1016/j.neuroimage.2016.08.016
7. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78. Available from: http://dx.doi.org/10.1016/j.neuroimage.2015.10.019
8. Baron CA, Kate M, Gioia L, Butcher K, Emery D, Budde M, et al. Reduction of Diffusion-Weighted Imaging Contrast of Acute Ischemic Stroke at Short Diffusion Times. Stroke. 2015;46(8):2136–41.
9. Budde MD, Frank JA. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc Natl Acad Sci U S A. 2010;107(32):14472–7.
10. Rahman N, Xu K, Arezza N, Borsos K, Budde M, Brown A, et al. Microstructural Diffusion MRI in Mouse Models of Severe and Repetitive Mild Traumatic Brain Injury. In: Proceedings of the 30th Annual International Symposium of Magnetic Resonance in Medicine. 2021. p. 11–3.
11. Skinner NP, Kurpad SN, Schmit BD, Budde MD. Detection of acute nervous system injury with advanced diffusion-weighted MRI: A simulation and sensitivity analysis. NMR Biomed. 2015;28(11):1489–506.
12. Zhuo J, Xu S, Hazelton J, Mullins R, Simon J. Diffusion Kurtosis as an In Vivo Imaging Marker for Reactive Astrogliosis in Traumatic Brain Injury. Nueroimage. 2012;59(1):467–77. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf
13. Szczepankiewicz F, Lasič S, van Westen D, Sundgren PC, Englund E, Westin CF, et al. Quantification of microscopic diffusion anisotropy disentangles effects of orientation dispersion from microstructure: Applications in healthy volunteers and in brain tumors. Neuroimage. 2015;104:241–52.
14. Nilsson M, Szczepankiewicz F, Brabec J, Taylor M, Westin CF, Golby A, et al. Tensor-valued diffusion MRI in under 3 minutes: an initial survey of microscopic anisotropy and tissue heterogeneity in intracranial tumors. Magn Reson Med. 2020;83(2):608–20.
15. Andersen KW, Lasič S, Lundell H, Nilsson M, Topgaard D, Sellebjerg F, et al. Disentangling white-matter damage from physiological fibre orientation dispersion in multiple sclerosis. Brain Commun. 2020;2(2).
16. Zhou M, Stobbe R, Szczepankiewicz F, Lloret M, Buck B, Fairall P, et al. Tensor-valued Diffusion MRI Shows Elevated Microscopic Anisotropy and Tissue Heterogeneity in White and Grey Matter of Acute Ischemic Stroke. In: Proc Intl Soc Mag Reson Med. 2021.
17. Hammelrath L, Škokić S, Khmelinskii A, Hess A, van der Knaap N, Staring M, et al. Morphological maturation of the mouse brain: An in vivo MRI and histology investigation. Neuroimage. 2016;125:144–52.
18. Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27(44):11869–76.
19. van de Looij Y, Mauconduit F, Beaumont M, Valable S, Farion R, Francony G, et al. Diffusion tensor imaging of diffuse axonal injury in a rat brain trauma model. NMR Biomed. 2012;25(1):93–103.
20. Molina ISM, Salo RA, Abdollahzadeh A, Tohka J, Gröhn O, Sierra A. In vivo diffusion tensor imaging in acute and subacute phases of mild traumatic brain injury in rats. eNeuro. 2020;7(3):1–18.
Figures