0969
Enzymatic activity monitoring through Dynamic Nuclear Polarization in Earth magnetic field1CNRS, Bordeaux, France, 2CNRS, Marseille, France, 3INSERM, Bordeaux, France
Synopsis
Earth-field MRI can provide new contrasts leading to the observation of pathologies at the biochemical level. However detection sensitivity is poor at low-field. In a preliminary spectroscopic approach, it is proposed here to detect protease-driven hydrolysis of a nitroxide probe thanks to electron-nucleus Overhauser enhancement in a homemade double resonance system. The nitroxide probe is a six-line nitroxide whose lines are shifted according to its substrate/product state. The Overhauser enhancement frequency dependence was in agreement with theoretical calculations. Enzymatic conversion of the nitroxide substrate was observed which opens the way for the design of new low-field DNP-MRI systems.
Purpose
To monitor and report the enzymatic activity of neutrophil elastase through DNP in Earth's magnetic field (EFNMR-DNP) with a specific nitroxide.Introduction
In vivo imaging of proteolysis is an appealing promise in MRI. It would allow a better understanding of human physiology, early detection and prognosis of inflammation or cancer and therapy testing1. In parallel, Earth field NMR developments aim at providing cost-efficient and portable systems2. In order to tackle the issue of sensitivity, electron-to-proton Dynamic Nuclear Polarization3 (DNP) using protease-sensitive free radicals is therefore an opportunity.Subject & Method
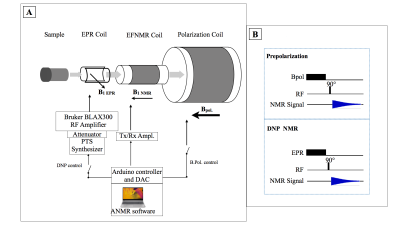
A six-line Succinyl-Ala-Ala-Pro-Val-nitroxide substrate4 (1.2 mM in 30 mL water) was used with or without neutrophil elastase (40 nM). The proteolysis product is a ketone with shifted Electron Resonance (EPR) lines. Overhauser enhancements at low-field were calculated (12 eigen states for the electron spin, 20 EPR transitions). The instrumental “Earth field-NMR” setup integrates (figure 1.A): 1) a magnetic prepolarization unit (switchable solenoid coil producing 13mT). 2) A DNP unit (RF transmitter, a 32mm RF Coil to saturate nitroxide EPR transitions in Earth’s field at about 150MHz). 3) An 1H NMR unit (transmit/receive channel at 2kHz ; a 50mm LF transmit-receive coil). 4) A programmable Arduino environment for hardware control and an open-source NMR software (ANMR)5. A classical pulse-FID sequence followed either pre-polarization at 13 mT or EPR saturation, with the following parameters: Tpol/TR = 500ms / 1000ms, NMR pulse length: 2.15 ms, Tacq : 3 min (figure 1.B).Results
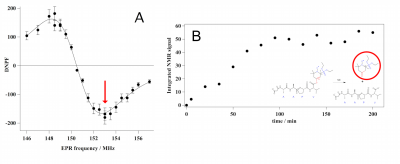
EFNMR-DNP acquisition allowed to measure the DNP enhancement spectrum of the proteolysis reaction product (ketone nitroxide, figure 2.A). Theoretical maximal and minimal enhancements (Dynamic Nuclear Polarization factor, DNPF) at 149MHz and 153MHz using linearly polarized wave were well predicted. The enzymatic reaction was observed by measuring the formation of the ketone product as a function of time through DNP at 153MHz EPR frequency (figure 2.B). A full conversion was observed after 2 hours.Discussion
The instrumental EFNMR-DNP setup allowed to properly measure Overhauser enhancements of a six-line nitroxide in Earth's field which were in agreement with theoretical prediction. Higher enhancements are anticipated to be produced with circularly polarized EPR saturation.Conclusion
Enzymatic conversion of the nitroxide substrate was unambiguously observed, opening the way of molecular imaging of proteolysis at low field in the future. Work is in progress to implement imaging modality to the EF system in order to investigate the feasibility of molecular imaging of inflammation.Acknowledgements
This Project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 863099References
1. Jugniot N, Voisin P, Bentaher A, Mellet P. Contrast Media Mol Imaging. Jun 12;2019:7417192. doi: 10.1155/2019/7417192.
2. Michal CA. J Magn Reson. 2020 Oct;319:106800. doi: 10.1016/j.jmr.2020.106800.
3. Overhauser A.W., Phys Rev 92, 411-415 (1953)
4. Jugniot N., Duttagupta I., Rivot A., Massot P., Cardiet C., Pizzoccaro A., Jean M., Vanthuyne N., Franconi J.M., Voisin P., Devouassoux G., Parzy E., Thiaudiere E., Marque S.R.A., Bentaher A., Audran G., Mellet P. Free Radic. Biol. Med.126 (2018) 101-112. https://doi.org/10.1016/j.freeradbiomed.2018.08.006
5. Michal, C. A. (2010). A low-cost spectrometer for NMR measurements in the Earth's magnetic field. Measurement Science and Technology, 21(10), 105902.
Figures