0968
Female patients with atopic dermatitis have reduced functional connectivity in the precuneus and inferior parietal lobe1Karolinska Institutet, Stockholm, Sweden, 2Division of Dermatology and Venereology, Karolinska Institutet, Stockholm, Sweden, 3Department of Biomedical and Clinical Science, Linköping University, Linköping, Sweden, 4CLINTEC, Karolinska Institutet, Stockholm, Sweden
Synopsis
A cohort of 32 atopic dermatitis (AD) patients and 32 matched healthy controls (HC) were recruited to study the neurological abnormalities which might be associated with this pruritic skin condition. The main findings are 1) reduced general functional connectivity in the precuneus, inferior parietal lobe and visual cortex was detected in females AD patients; 2) Severity of the symptoms was negatively correlated with functional connectivity in inferior parietal lobe, while salivary cortisol was positively associated. Resting-state fMRI can provide useful insight into the altered neurophysiology and clinically relevant assessment for the AD disorder.
Introduction
Atopic dermatitis (AD), is a chronic inflammatory skin disorder, characterized by a dry and pruritic skin condition and has received little attention in the neuroimaging literature in spite of its clinical relevance. Previous studies[1,2] suggested that the itch perception is associated with brain activity response in the salience network for attention control and sensorimotor preparation for scratching. However, much is still unknown about the brain functional circuitries affected by the highly debilitating AD. The purpose of this study is to investigate the brain functional connectivity differences between well-characterized AD patients in comparison with matched controls, as well as the possible association of the altered functional connectivity with clinical and laboratory parameters.Methods
A total of 32 adult subjects(f/m=17/15) with a clinical diagnosis of AD and a score greater than 30 on the SCORing Atopic Dermatitis (SCORAD) scale were enrolled. 32 matched health controls were also included. An array of 20 different clinical and laboratory measurements were performed, including objective and subjective degrees of the eczema, salivary cortisol, level of itching, somatic predisposition, and neuropsychological assessments of stress, fatigue, irritability, impulsivity, anxiety, depression, aggressiveness, and assertiveness. For implication a principal component analysis (PCA) of the clinical data was conducted. The MRI data acquisition was conducted on a whole-body 3T MRI scanner (Magnetom Trio, Siemens) equipped with a 32-channel receiving head coil. Besides routine clinical sessions, each subject completed a R-fMRI session. The acquisition parameters included the following: TE/TR=35/2500 ms, flip angle=90°, 39 slices of 4.5 mm thick, FOV=230 mm, matrix=96×96, GRAPPA iPAT=2, and 190 dynamic timeframes. The R-fMRI datasets underwent a standard preprocessing procedure[3] with a bash wrapper calling programs from AFNI and FSL packages. After the preprocessing, resting-state function connectivity (RFC) metrics were calculated using a quantitative data-driven analysis (QDA) framework[4,5], which derives two types of threshold-free RFC metrics: the connectivity strength index (CSI) and connectivity density index (CDI) assessing the local functional connectivity with rest of the brain. The negative and positive portions were separated and denoted with the subscript “N” and “P”, respectively, to avoid information cancelation.The statistical significance of the t-test and regression analysis was assessed by using a two-step approach. Firstly, a voxel-wise threshold p<0.001 (t-score ≥3.532) was imposed to form the initial cluster candidates. Then permutation simulations were performed to identify the brain regions of interest (ROI) out of the initially detected clusters at family-wise error rate (FWER) p≤0.05. Using the detected ROIs as masks, the mean time courses of the BOLD signals were used to calculate the ROI specific functional connectivity maps.Results
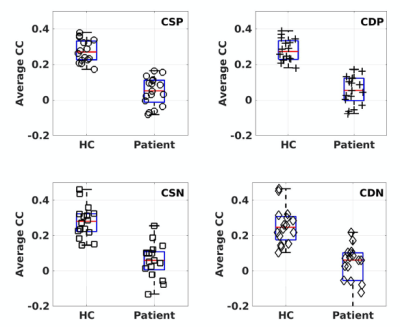
The first 3 PCA components accounted for 91.9% of the total variance (the contributions were 66.0, 16.6. and 9.3%, respectively). The SCORAD estimates and salivary cortisol measurements are the main positive contributors for the 2 top PCA components, respectively (See Figure 1). The two-sample t-test results of the RFC metrics derived from QDA revealed 3 ROIs with significantly altered RFC (FWER, p<0.05) in female AD patients in comparison with the corresponding HC subjects (Figure 2). The RFC difference between AD and HC subjects become statistically insignificant when all the subjects (male and females) are included. The specific connectivity between the prefrontal cortex and the seed ROI in the precuneus (as detected by the two-sample t-test of the QDA results) is significantly reduced in female AD patients compared to the HC subjects, irrespective of the type of RFC metrics (CDIP, CSIP, CDIN or CSIN) used to define the seed ROI (Figure 3)Both the CSIP and CDIP metrics showed significant correlation with the top two PCA components in a number of brain regions. Figure 4 show the regression results for CSIP and CDIP versus PCA1. As summarized in Table 2, PCA1 is negatively correlated with CDIP and CSIP in R-inferior parietal lobe, L-angular gyrus, R-postcentral gyrus and insular lobe. PCA1 is also positively correlated with CDIP and CSIP in the L-precentral gyrus. Details of the ROIs are summarized in Table 2. Similarly, the regression results for PCA2 are summarized in Table 3.Discussion
As reviewed by Pfab et al.[6], a number of studies in healthy subjects have experimentally demonstrated that induced itch activates a brain network including motor network, cingulate, insular, IPL, and prefrontal cortices. A few studies involving AD patients have also suggested that the responses to evoked itch are different between HC and AD patients [1,2,7-9]. This is consistent with the main findings of this study regarding the abnormal functional connectivity in female AD patients. Results from both QDA and seed-based analysis indicate that patients with higher degree of the eczema severity is correlated with a decrease in general functional connectivity in precuneus, R-IPL, and specific connectivity between prefrontal and precuneus. Moreover, both the regression analysis and t-test results have demonstrated the similar trend that the brain networks implicated in itch provocation studies[8] are affected by the AD symptoms in a complicated pattern. Our study clearly demonstrates for the first time that “resting” functional connectivity is altered in female AD patients and these state-specific alterations can be directly linked with different aspects of the symptom severity.Conclusion
Resting-state fMRI can provide useful insight into the altered neurophysiology and clinically relevant assessment for the AD disorder.Acknowledgements
No acknowledgement found.References
1. Desbordes G, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, Pfab F, Napadow V. Evoked itch perception is associated with changes in functional brain connectivity. Neuroimage Clin 2015;7:213-221. 2. Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol 2009;161(5):1072-1080. 3. Li TQ, Wang Y, Hallin R, Juto JE. Resting-state fMRI study of acute migraine treatment with kinetic oscillation stimulation in nasal cavity. Neuroimage Clin 2016;12:451-459. 4. Li X, Fischer H, Manzouri A, Månsson KNT, Li T-Q. A Quantitative data-driven analysis framework for resting-state functional magnetic resonance imaging: A study of the impact of adult age. Front Neurosci 2021;15. 5. Li X, Fischer H, Manzouri A, Mansson KNT, Li TQ. Dataset of whole-brain resting-state fMRI of 227 young and elderly adults acquired at 3T. Data Brief 2021;38:107333. 6. Pfab F, Valet M, Napadow V, Tölle TR, Behrendt H, Ring J, Darsow U. Itch and the brain. Chem Immunol Allergy 2012;98:253-265. 7. Schneider G, Stander S, Burgmer M, Driesch G, Heuft G, Weckesser M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur J Pain 2008;12(7):834-841. 8. Napadow V, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, Pfab F. The brain circuitry mediating antipruritic effects of acupuncture. Cereb Cortex 2014;24(4):873-882. 9. Mochizuki H, Kursewicz CD, Nomi JS, Yosipovitch G. The right default mode network is associated with the severity of chronic itch. J Eur Acad Dermatol Venereol. 2021;35(11):e819-e821.
Figures