0961
Metal Artifact Corrected and Chemical Shift Encoded MRI
Collin J Buelo1,2, Timothy J. Colgan1, and Diego Hernando1,2
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Fat-suppressed MRI near metal remains a challenging technical problem. Chemical shift encoded (CSE) water/fat separation enables excellent SNR efficiency compared to short tau inversion recovery (STIR), but the large B0 field offsets and gradients complicate conventional CSE methods near metal. To address these challenges, we propose metal artifact correction and chemical shift encoding using optimized echo spacings, and with deep learning processing for reconstruction of water/fat separated imaging near metal. This method is able to correctly separate water and fat closer to a metallic surgical breast clip than conventional methods, without the drawbacks associated with STIR imaging.
Introduction
Fat-suppressed MRI near metal remains a challenging technical problem. Short tau inversion recovery (STIR) is a method of fat suppression can be robust to the large metal-induced B0 offsets, however, STIR severely reduces signal-to-noise ratio (SNR) and limits the achievable image contrasts[1,2].Chemical shift encoded (CSE) water-fat separation enables excellent SNR efficiency compared to STIR, but the large B0 field offsets and gradients complicate conventional CSE methods near metal. Traditional two-point spin echo Dixon imaging acquires an on-resonance echo, where water and fat are in phase, and an additional echo shifted by , where is the frequency shift between the water peak and the main fat peak (e.g. $$$\Delta f=434$$$Hz at 3.0T)[3]. However, the high B0 gradients observed near metal lead to dephasing of the acquired signals, with rapidly growing metal-induced signal void as a function of increasing echo shift. Therefore, shorter echo spacings may be advantageous near metal. Further, traditional regularized CSE water-fat processing relies on assumptions of B0 smoothness, which may not be appropriate neat metal.
To address these challenges, we propose metal artifact correction and chemical shift encoding (MacnChese), a method of acquiring multi-spectral MRI with chemical shift encoding using optimized echo spacings, and with deep learning processing for reconstruction of water-fat separated imaging near metal.
Methods
Surgical Breast ClipThis method was developed for imaging near a metallic clip soft tissue marker (SmartClip™, Elucent Medical, Eden Prairie, MN) used in breast surgery for tumor localization. Previous work has measured the magnetization of this clip to be $$$ 1.14\cdot 10^{-4} A\cdot m^2 $$$ at 3.0T[4]. This magnetization was used to generate the field map around the clip for training data simulation.
Pulse Sequence
This work relied on a multi-spectral imaging method (MAVRIC) for imaging near metal. MAVRIC utilizes multi-spectral acquisition and view angle tilting to reduce the metal artifact[5][6]. In this work, the MAVRIC acquisition was modified to obtain data at shifted echo times to encode chemical shift into the acquired images. Echoes were acquired at [0,296,596,1188]$$$\mu s$$$. The shorter echo shifts (0, 296, and 596$$$\mu s$$$) preserve signal near the metal, while the longer echo shift (1188$$$\mu s$$$, i.e., opposed-phase at 3.0T) is the common choice for SNR-optimal water-fat separation.
Synthetic Data Simulation
A segmented 3D breast MRI was used to simulate a MacnChese acquisition[7]. The segmented scan was input into a data augmentation pipeline to generate varied training data[8]. Additional detail is shown in Figure 1.
Synthetic MacnChese data were generated for training(see Figure 2). After simulation of multi-echo MacnChese images, a randomly oriented linear 3D gradient was used to scale the image amplitude to simulate coil sensitivity variation.
Network Training
The simulated data described above was then used to train a 3D U-Net, with the ground truth water and fat maps as the output. The network was trained with 100 128x128x24 image volumes. Using Tensorflow with the Adam optimizer, 2000 epochs with 10 samples per batch, training took 8 hours using an NVIDIA Ampere A100 GPU.
Test Phantom
A test phantom was constructed by pouring an agar-based gel into a container, then after curing, cutting this gel into irregular shapes, and placing them in a 11x12x11cm3 container. Peanut oil was then poured into this container to cover the gel pieces. This created a structure resembling that of in-vivo breast tissue, with irregular boundaries between tissue and fat.
Imaging
The phantom was imaged at 3.0T (Signa PET/MR, GE Healthcare), with MacnChese, as well as a three-dimensional spoiled gradient recalled echo (SGRE) 6-echo CSE sequence and a 3D fast spin echo (FSE) 2-echo CSE sequence, to compare processing to standard water-fat separation algorithms. Voxel dimensions for all acquisitions were 1.875x1.875x5mm3 with a matrix size of 128x128x24.
Results
The MacnChese acquisition time was 24 minutes, done in two separate acquisitions due to limitations in raw data storage. A visualization of the image processing pipeline is shown in Figure 4. Reconstruction results for the MacnChese acquisition as well as SGRE(6-echo) and FSE(2-echo) CSE water-fat reconstructions are shown in Figure 5. While artifact sizes are similar between MacnChese and the conventional CSE methods, there is substantially less swapping in the MacnChese image, where there is no fat signal visible within the gel.Discussion
This work demonstrates a chemical shift encoded method for imaging near metal. Shifted echoes were acquired with a multispectral acquisition and fed into a deep neural network for water-fat separation. This method was able to correctly separate water and fat closer to a metallic surgical breast clip than conventional methods, without the drawbacks associated with STIR imaging. While this method was developed and evaluated on a metallic surgical breast clip, it can be adapted to other metallic implants.This work has several limitations. It is a preliminary evaluation of a novel method. Further optimization of both acquisition and reconstruction are needed. Clinically used MAVRIC is a highly accelerated method to achieve scan times of a few minutes, however no acceleration was used in the MacnChese acquisition. Significant reductions in scan time can be obtained through use of parallel imaging and further pulse sequence optimization. Acquired echo times may be optimized to reconstruct closer to the metal while maintaining water-fat separation.
Acknowledgements
The authors wish to thank Elucent Medical for providing funding for this study, as well as Leah Henze Bancroft for providing a segmented breast scan use for phantom generation. In addition, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, we would like to thank Dr. Suryanarayanan Kaushik from GE Healthcare for assistance with the reconstruction of MAVRIC SL data.References
[1] P. M. Jungmann, C. A. Agten, C. W. Pfirrmann, and R. Sutter, “Advances in MRI around metal,” J. Magn. Reson. Imaging, vol. 46, no. 4, pp. 972–991, 2017, doi: 10.1002/jmri.25708.[2] M. R. Smith, N. S. Artz, C. Wiens, D. Hernando, and S. B. Reeder, “Characterizing the limits of MRI near metallic prostheses: Limits of MRI Near Metallic Prostheses,” Magn. Reson. Med., vol. 74, no. 6, pp. 1564–1573, Dec. 2015, doi: 10.1002/mrm.25540.
[3] Q.-S. Xiang, “Two-point water-fat imaging with partially-opposed-phase (POP) acquisition: An asymmetric Dixon method,” Magn. Reson. Med., vol. 56, no. 3, pp. 572–584, 2006, doi: 10.1002/mrm.20984.
[4] C. J. Buelo, S. B. Reeder, and D. Hernando, “Systematic characterization of MRI near a surgical breast implant at 1.5T and 3.0T,” presented at the ISMRM, 2021.
[5] K. M. Koch et al., “Imaging near metal with a MAVRIC-SEMAC hybrid,” Magn. Reson. Med., vol. 65, no. 1, pp. 71–82, 2011, doi: 10.1002/mrm.22523.
[6] S.-J. Choi, K. M. Koch, B. A. Hargreaves, K. J. Stevens, and G. E. Gold, “Metal Artifact Reduction With MAVRIC SL at 3-T MRI in Patients With Hip Arthroplasty,” AJR Am. J. Roentgenol., vol. 204, no. 1, pp. 140–147, Jan. 2015, doi: 10.2214/AJR.13.11785.
[7] L. Henze, C. Moran, and F. Kelcz, “Digital breast phantom for evaluating dynamic accelerated imaging methods,” presented at the ISMRM, Stockhold, Sweden, 2010.
[8] Billot B, Robinson E, Dalca AV, Iglesias JE, editors. Partial Volume Segmentation of Brain MRI Scans of Any Resolution and Contrast2020; Cham: Springer International Publishing.
Figures
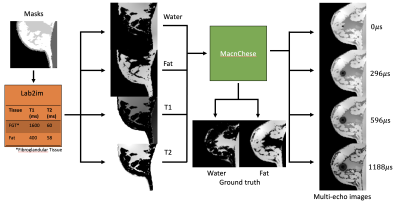
Figure 1: Tissue segmentations from a
digital phantom are fed into Lab2im[8], a data augmentation toolbox. This deforms
the source masks and assigns values for different tissues for each output
parameter. T1 and T2 values for fibroglandular
tissue and fat are shown in the orange box. Parameter maps are then fed into a MacnChese
acquisition simulation, from which multi-echo and ground truth water/fat images
are created.
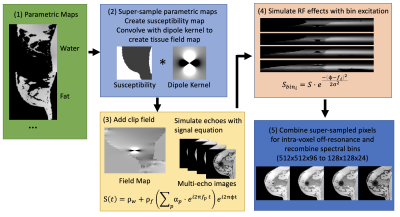
Figure 2: MacnChese acquisition simulation: (1) Supersample
parametric maps to simulate intra-voxel off-resonance (2) Create susceptibility
map (water = 0, fat = 2, air = 9 ppm), convolve to create field map (3) Add
clip field and simulate multi-echo images with spin echo signal equation (4) RF
effects are simulated with gaussian spectral bin profile excitation ($$$f_i$$$=frequency
of bin $$$i$$$, σ=bin
spectral width) (5) Pixels are combined to simulate intra-voxel off-resonance,
and the spectral bins are recombined.
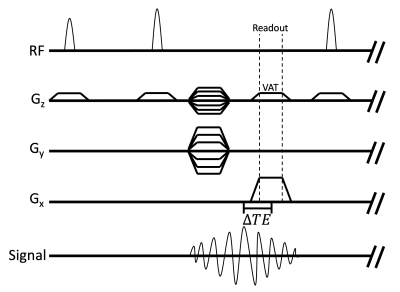
Figure 3: MacnChese pulse sequence diagram. MacnChese is a
3D FSE acquisition that utilizes multi-spectral imaging and view angle tilting
(VAT) to image near metal, while also acquiring shifted echoes for chemical
shift encoding. Phase data is lost when spectral bins are combined. The VAT
pulse is the same amplitude as the slab selection gradient, which corrects slab
excitation distortion in readout.
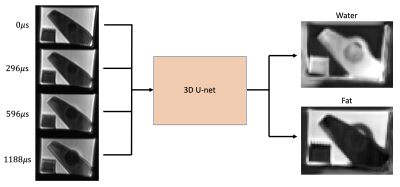
Figure 4: MacnChese image reconstruction. The four echo
images are fed into the trained 3D U-net, with water and fat images as output.

Figure 5: Reconstructed water and fat images from the three methods tested. Artifact size is similar between the three methods, however MacnChese suffers less from swaps surrounding the metal. Very little fat is reconstructed close to the clip, and the boundary between gel and fat remains visible near the clip.
DOI: https://doi.org/10.58530/2022/0961