0955
Near Metal MRI with Compressed Sensing PETRA1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Department of Diagnostic and Interventional Radiology, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 3Department of Orthopaedics and Trauma Surgery, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
MRI near metallic implants require high bandwidth excitation and acquisition to minimize signal void and pile-up artefacts. In this work, compressed sensing PETRA (csPETRA) was modified to have intentionally a longer TE, so that the phase-encoding part of the k-space is extended. To prevent extremely long scan times, SPI points in csPETRA sequence are pseudo-randomly undersampled to reduce the scan time below 6 minutes. csPETRA reduced artifacts near metal significantly while preserving the anatomical details. Isotropic 3D MRI of two volunteers with mouth and knee prostheses was performed with csPETRA and the artifacts are significantly reduced compared to T1w-WARP sequence.
Introduction
Magnetic susceptibility differences between metallic implants and tissue cause magnetic field perturbations up to 5000ppm that result in signal voids and image distortion artifacts due to intra-voxel dephasing and image displacements. To mitigate this problem several techniques have been proposed including Single Point Imaging(SPI)1, slice encoding for metal artifact correction(SEMAC)2, multi-acquisition variable resonance image combination(MAVRIC)3, and view angle tilting(VAT)4.Fully phase-encoded techniques such as SPI offer distortion-free near metal imaging5-7. As SPI is intrinsically time-inefficient, these methods cannot provide 3D isotropic high-resolution(≤1mm) images in clinically feasible times (≤6minutes). To overcome this time penalty, hybrid sequences such as PETRA8-10 have been proposed which acquire a central k-space region using SPI, and the other k-space with a radial acquisition.
To increase the acquisition bandwidth in PETRA, the frequency‑encoding gradients are already switched on before the RF excitation. The finite RF pulse duration and acquisition delays between RF pulse and data acquisition result in a sampling gap at the center of k-space and this missing gap is recovered using SPI.
In this work, we used compressed sensing PETRA (csPETRA)11 with high receiver bandwidth and a TE that is longer than the dead time of the MR system to extend the SPI part of the sequence. To prevent extremely long scan times, SPI points in csPETRA sequence are pseudo-randomly undersampled using Poisson disk sampling12 reducing the scan time below 6 minutes.
Methods
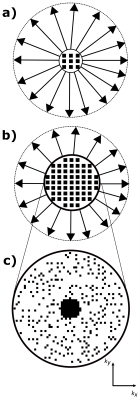
PETRA consists of an outer radial k-space acquisition and an SPI section to fill the inner k-space gap (Fig. 1). The required number of SPI samples is approximately $$$N_{spi}=\frac{4}{3}\pi(N_{gap})^3$$$ $$$(N_{gap}=\frac{TE}{t_{dwell}}$$$, $$$t_{dwell}$$$: dwell time$$$)$$$, which shows a cubic relation between the radius of the gap and total scan time. To reduce the scan time for the SPI section only a small portion of the SPI points is acquired in csPETRA, and the image is reconstructed by solving the following minimization problem:$$\begin{matrix}\underset{\mathbf{m}}{\operatorname{argmin}}\lambda\text{TV}(\mathbf{m})+\alpha\|\mathbf{m}\|_1\\s.t.\|\mathbf{M\mathcal{F}m}-\mathbf{s}\|_2<\epsilon\end{matrix},$$where $$$\mathbf{M}$$$ is the sampling mask, $$$\mathbf{m}$$$ is the vectorized 3D image, $$$\mathbf{\mathcal{F}}$$$ is the 3D discrete Fourier transform operator, TV(⋅) is 3D total variation operator, $$$\|⋅\|_1$$$ is $$$\ell_1$$$-norm operator, $$$\|⋅\|_2$$$ is $$$\ell_2$$$-norm operator, $$$\lambda$$$ and $$$\alpha$$$ are scalar weights of TV and $$$\ell_1$$$-norm regularization operators, respectively. $$$\epsilon$$$ is the bound on the data fidelity error. The CS reconstruction was implemented using Alternating Direction Method of Multipliers13 of the BART toolbox14.In this study, we increased TE together with the acceleration factor(Acc) of SPI imaging reducing the scan time below 6 minutes. As a result, the number of phase-encoded samples is increased(Fig. 1c). The performance of the method is compared to the WARP (Siemens, Erlangen, Germany) sequence, which is based on high bandwidth TSE and VAT technique4.
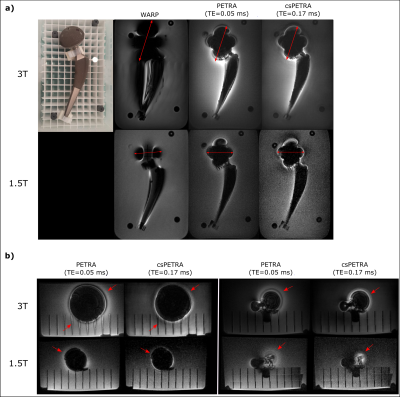
A phantom was constructed using a clinical hip prosthesis (Aesculap AG, Tuttlingen, Germany; Fig. 2) consisting of porcelain and titanium alloy. Phantom experiments were performed at 1.5T(Aera, Siemens, Erlangen) and 3T MRI systems (Prisma Fit, Siemens, Erlangen) using a 4‑channel flex coil for signal reception. In-vivo data of the maxilla and the knee were acquired of two volunteers with metallic orthopaedic implants using T1w-WARP, PETRA, and csPETRA sequences and a 20-ch head coil and 15-ch Tx/Rx knee coil (QED, Cleveland, OH), respectively. PETRA/csPETRA acquisition parameters were as follows: (1x1x1mm)3 voxel size, TR=2ms, TE=0.05/0.17-0.18ms, tacq=6min, , and $$$\alpha$$$=3°. Imaging parameters of WARP at 1.5T/3T: TR=1390ms, TE=5.8/6.1ms, FOV=256x256mm, voxel size=(1x1x2)mm3, $$$\alpha$$$=150°, ETL=8.
System reported time averaged RF power and whole-body SAR of WARP/csPETRA sequences at 1.5T was 99/19.3Watt and 1.7/0.3Watt/kg, whereas they were 25.4/7.1Watt and 1.9/0.6Watt/kg at 3T.
Results
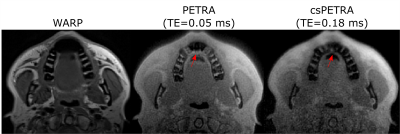
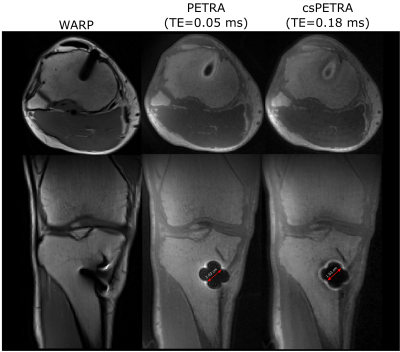
In Fig. 2 WARP and PETRA images are shown at 1.5T and 3T together with a photograph of the implant. PETRA and csPETRA outperformed the WARP technique in terms of the size of the near metal artifacts. In 3T images of WARP, PETRA and csPETRA, diameter of the artifacts at the head and acetabular component of the hip prosthesis was 10.9cm, 8cm, and 6.6 cm, respectively. csPETRA method has further reduced ringing and signal pile-up artifacts for about 10% compared to PETRA(red arrows, Fig. 2). At 1.5T, phantom image of csPETRA had spatially heterogeneous signal intensity(Fig 2a).In Fig. 3, in-vivo WARP, PETRA, and csPETRA transverse slices of the maxilla are presented. The PETRA-based methods showed substantial improvement over WARP technique in terms of artifacts. Sizes of the signal void artifacts at WARP, PETRA, and csPETRA images were 3cm, 2.2cm, and 1.5cm, respectively. csPETRA with TE=0.18ms showed minimum artifact, e.g., incisor teeth of the maxilla were made partly visible (red arrows, Fig. 3). In Fig. 4, knee images of a volunteer with a metallic screw for graft fixation in anterior cruciate ligament reconstruction at the tibia is presented. csPETRA images were slightly blurrier compared to PETRA images but SNR was only 5% lower despite significant decrease of effective acquisition time.
Discussion
csPETRA reduces artifacts near metal significantly while preserving the anatomical details in clinically relevant scan times(≤6 minutes). With csPETRA, short-T2 isotropic 3D MRI of the maxilla and knee with metallic prosthesis was possible. Another advantage of the csPETRA is 3-fold lower SAR deposition compared to SE-based techniques in expense of the soft tissue contrast. Magnetization preparation pulses and longer pulse durations with higher flip angles can be applied to improve contrast.Acknowledgements
.References
1. Ramos-Cabrer P, van Duynhoven JPM, Van der Toorn A, Nicolay K. MRI of hip prostheses using single-point methods: in vitro studies towards the artifact-free imaging of individuals with metal implants. Magn. Reson. Imaging 2004;22:1097–1103 doi: 10.1016/j.mri.2004.01.061.
2. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn. Reson. Med. 2009;62:66–76 doi: 10.1002/mrm.21967.
3. Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn. Reson. Med. 2009;61:381–390 doi: 10.1002/mrm.21856.
4. Cho ZH, Kim DJ, Kim YK. Total inhomogeneity correction including chemical shifts and susceptibility by view angle tilting. Med. Phys. 1988;15:7–11 doi: 10.1118/1.596162.
5. Wiens CN, Artz NS, Jang H, McMillan AB, Koch KM, Reeder SB. Fully phase‐encoded MRI near metallic implants using ultrashort echo times and broadband excitation. Magn. Reson. Med. 2018;79:2156–2163 doi: 10.1002/mrm.26859.
6. Artz NS, Hernando D, Taviani V, Samsonov A, Brittain JH, Reeder SB. Spectrally resolved fully phase-encoded three-dimensional fast spin-echo imaging. Magn. Reson. Med. 2014;71:681–690 doi: 10.1002/mrm.24704.
7. van Gorp JS, Bakker CJG, Bouwman JG, Smink J, Zijlstra F, Seevinck PR. Geometrically undistorted MRI in the presence of field inhomogeneities using compressed sensing accelerated broadband 3D phase encoded turbo spin-echo imaging. Phys. Med. Biol. 2015;60:615–631 doi: 10.1088/0031-9155/60/2/615.
8. Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn. Reson. Med. 2012;67:510–518 doi: 10.1002/mrm.23017.
9. Hilgenfeld T, Prager M, Heil A, et al. PETRA, MSVAT-SPACE and SEMAC sequences for metal artefact reduction in dental MR imaging. Eur. Radiol. 2017;27:5104–5112 doi: 10.1007/s00330-017-4901-1.
10. Kobayashi N, Goerke U, Wang L, Ellermann J, Metzger GJ, Garwood M. Gradient-Modulated PETRA MRI. Tomography 2015;1:85–90 doi: 10.18383/j.tom.2015.00157.
11. Ilbey S, Fischer J, Bock M, Ozen AC. Compressed Sensing PETRA MRI. In: Proceedings of the 29th scientific meeting, International Society for Magnetic Resonance in Medicine, Virtual; p. 908.
12. Cook RL. Stochastic sampling in computer graphics. ACM Trans. Graph. 1986;5:51–72 doi: 10.1145/7529.8927.
13. Boyd S, Parikh N, Chu E. Distributed optimization and statistical learning via the alternating direction method of multipliers. Now Publishers Inc; 2011.
14. Tamir JI, Ong F, Cheng JY, Uecker M, Lustig M. Generalized magnetic resonance image reconstruction using the Berkeley advanced reconstruction toolbox. In: ISMRM Workshop on Data Sampling & Image Reconstruction, Sedona, AZ. ; 2016.
Figures