0954
Improvements of Image Quality of 1H and 129Xe MRI by Using an Advanced Acquisition and Reconstruction Method Coupled with Deep Learning1Physics and Astronomy, The University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3School of Biomedical Engineering, The University of Western Ontario, London, ON, Canada
Synopsis
Accelerated MRI has the potential to significantly improve image quality without increasing costs, especially for low field strengths. A series of undersampled images are averaged for every unique permutation, and their SNR dependency is fitted to the Stretched-Exponential-Model. The proposed method was implemented in proactively undersampled phantom images at low field (0.074T) and in retroactively undersampled human lung images at high field (3T) using the FGRE pulse sequence; in all cases, SNR was significantly improved within the same scan duration compared to a fully-sampled image. Reconstruction artefacts were minimized or completely removed using a convolutional neural network.
Introduction
MRI is largely limited by the amount of magnetizable nuclei and their gyromagnetic ratios1. Traditional approaches to remedy this low sensitivity, such as higher field strength and enriched contrast agents (13C),2 often incur additional material and operational-costs. In certain cases, such as hyperpolarized gas lung MRI with naturally-abundant 129Xe,3 this low-sensitivity issue still poses considerable problems.It has recently been shown that accelerated MRI via Compressed-Sensing, combined with fitting to the Stretched-Exponential-Model, is able to provide considerable gains in SNR without increasing total scan-duration.4
To reduce the k-space coverage needed to construct an adequate image (and therefore the sampling time), k-space is undersampled according to high acceleration factors (AF): for example, an acceleration factor of 10 corresponds to acquiring 10% of k-space, resulting in 10x faster acquisition. It follows that acquiring a series of ten images, each undersampled to an AF of 10, should take as much time as a single fully-sampled image; these images can then be averaged together using a specific averaging pattern to yield significantly higher SNR. Specifically, if the SNR of this set of images is assumed to reflect the spin-density difference instead of the signal-level,4 then the SNR dependency of these images can be fitted to the Stretched-Exponential-Model equation.5-8 The feasibility of this approach was demonstrated using retroactively undersampled phantom images.4 One potential issue associated with this approach is the presence of image artefacts caused by reconstruction of the accelerated k-spaces. We hypothesize that a convolutional-neural-network capable of minimizing these artefacts from the reconstructed images9 can be developed using recent advancements in deep learning.10,11
In this proof-of-concept study, we proactively acquired accelerated proton resolution-phantom images at low-field and non-accelerated 129Xe human lung images using a small xenon dose at 3T to demonstrate this method’s potential in human subjects and its ability to reduce image reconstruction artefacts in phantoms.
Methods
Proton MR was performed on a resolution phantom at 0.074T using the Fast-Gradient-Recalled-Echo (FGRE) pulse sequence. Nine undersampled k-spaces were acquired for three acceleration-factors (AF): 7, 10, and 14. The following parameters were used: FOV=10x10cm2, matrix=128x128, TE/TR=1ms/3sec, BW=32kHz, Flip-Angle = 90°.These were subsequently averaged together for every unique combination of images without overlap (e.g., 2 combinations of 4 averages, 3 combinations of 3 images, etc.); the SNR dependency of the resulting 14 k-spaces was plotted as a function of the image number and fitted to the Stretched-Exponential-Model using the Abascal method.4,6
One healthy-volunteer with written informed consent, provided to an ethics-board-approved study protocol, underwent spirometry and 129Xe MRI scanning. Nine fully-sampled 129Xe human-lung images were acquired at 3.0T (MR750, GEHC) using whole-body-clinical-gradients and a commercial, xenon-quadrature-flex human RF-coil12 (MR Solutions). A centric 2D FGRE sequence for single 15mm coronal-slices (TE=2msec, TR=5msec, reconstructed matrix size=128x128, and FOV=40x40cm2, constant-flip-angle=4o, BW=16kHz) was utilized. All images were acquired in breath-hold (<16 second) after inspiration of 1.0L of gas (129Xe/4He mixture, 10/90) from functional-residual-capacity. Hyperpolarized 129Xe gas (polarization=35%) was obtained from a turn-key, spin-exchange-polarizer system (Polarean-9820 129Xe polarizer).13
The same aforementioned averaging scheme was applied, and the SNR dependency of the resulting 14 k-spaces (Fig.1) was plotted as a function of the image number (Fig.2). The SNR decay was assumed to represent the decreasing gas density of 129Xe after each wash-out oxygen breath.14,15
The 14 k-spaces were retroactively under-sampled for the same three AFs and for three sampling schemes (FGRE, x-Centric16, and FE-Sectoral17) and fitted to the Stretched-Exponential-Model as previously described.4,6
To minimize or remove the reconstruction artefacts, it was assumed that the reconstructed images were a superposition of the original (artefact-free) images and the artefacts themselves9. A 3-stage U-Net18 was developed9, and the artefact masks of 202 unrelated previously acquired phantom-images were generated and used as training data for the network (image-size=128x128, 202 images).
Results
Fig.1 shows averaged human lung images (highest SNR of 20.0) and the original non-averaged image (SNR=6.6). Fig.2 suggests that the signal attenuation can be fitted with the Stretched-Exponential-Model. Fig.3 depicts human-lung image reconstructions for all three sampling schemes and AFs; Frequency-Encoding-Sectoral showed minimal artefacting compared to FGRE and x-Centric (SNR>21 for all). Fig.4 shows reconstructed phantom images for the three acceleration factors, with severe artefacts and image degradation at higher AFs. Fig.5 shows the phantom reconstructions of Fig.4 before and after applying the neural network.Discussion and Conclusion
The proactively undersampled phantom images yielded significant SNR gains during the same total imaging time as the fully-sampled image, albeit with considerable artefacts at higher acceleration-factors. For the first time, we’ve demonstrated that the human lung acquisition/reconstructions can result in the large SNR improvement when using a third of the normal 129Xe dose. The artefact removal neural network was able to remove artefacts in the AF=7 phantom reconstruction, but performance suffered at higher AFs. This sampling and reconstruction method could be implemented in existing MRI setups as it does not require additional hardware; when paired with a pulse-sequence featuring minimal artefacts (such as Frequency-Encoding-Sectoral), this technique could become a valuable clinical-tool for diagnosing various pulmonary-diseases, with significant benefits in low-field systems.Acknowledgements
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada, R5942A04.
References
- Song, X.-x., Liu, Z.-j., Xu, X.-z. & Tang, Q. Highly sensitive MRI contrast agent for enhanced visualization of tumors. New J. Chem. 38, 3813-3818, doi:10.1039/c4nj00183d (2014).
- Thind, K. et al. Detection of radiation-induced lung injury using hyperpolarized (13)C magnetic resonance spectroscopy and imaging. Magn Reson Med 70, 601-609, doi:10.1002/mrm.24525 (2013).
- Stewart, N. J., Norquay, G., Griffiths, P. D. & Wild, J. M. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized three-dimensional steady-state free precession. Magn Reson Med 74, 346-352, doi:10.1002/mrm.25732 (2015).
- Perron, S., Fox, M. S., Serrai, H. & Ouriadov, A. Feasibility of a Novel Sampling/Reconstruction Method Ensuring a SNR Benefit Over the Traditional Sampling Approach. Proceedings of the 30th Annual Meeting of ISMRM, Vancouver, Canada (2021).
- Westcott, A., Guo, F., Parraga, G. & Ouriadov, A. Rapid single-breath hyperpolarized noble gas MRI-based biomarkers of airspace enlargement. J Magn Reson Imaging 49, 1713-1722, doi:10.1002/jmri.26574 (2019).
- Abascal, J., Desco, M. & Parra-Robles, J. Incorporation of Prior Knowledge of Signal Behavior Into the Reconstruction to Accelerate the Acquisition of Diffusion MRI Data. IEEE Trans Med Imaging 37, 547-556, doi:10.1109/TMI.2017.2765281 (2018).
- Berberan-Santos, M. N., Bodunov, E. N. & Valeur, B. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential). Chemical Physics 315, 171-182, doi:10.1016/j.chemphys.2005.04.006 (2005).
- Parra-Robles, J., Marshall, H. & Wild, J. M. Characterization of 3He Diffusion in Lungs using a Stretched Exponential Model [abstract]. ISMRM 21st Annual Meeting, 0820 (2013).
- Lee, D., Yoo, J. & Ye, J. C. Deep artifact learning for compressed sensing and parallel MRI. Magnetic Resonance in Medicine (2017).
- Yang, Y., Sun, J., Li, H. & Xu, Z. ADMM-CSNet: A Deep Learning Approach for Image Compressive Sensing. IEEE Transactions on Pattern Analysis and Machine Intelligence PP, 1-1, doi:10.1109/TPAMI.2018.2883941 (2018).
- Hammernik, K. et al. Learning a variational network for reconstruction of accelerated MRI data. Magnetic Resonance in Medicine 79, 3055-3071, doi:https://doi.org/10.1002/mrm.26977 (2018).
- Kaushik, S. S. et al. Single-breath clinical imaging of hyperpolarized (129)Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med 75, 1434-1443, doi:10.1002/mrm.25675 (2016).
- Kaushik, S. S. et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65, 1154-1165, doi:10.1002/mrm.22697 (2011).
- Ouriadov, A. V. et al. In vivo regional ventilation mapping using fluorinated gas MRI with an x-centric FGRE method. Magn Reson Med 74, 550-557, doi:10.1002/mrm.25406 (2015).
- Santyr, G. E., Lam, W. W. & Ouriadov, A. Rapid and efficient mapping of regional ventilation in the rat lung using hyperpolarized 3He with Flip Angle Variation for Offset of RF and Relaxation (FAVOR). Magn Reson Med 59, 1304-1310, doi:10.1002/mrm.21582 (2008).
- Ouriadov, A. V. & Santyr, G. E. High spatial resolution hyperpolarized (3) He MRI of the rodent lung using a single breath X-centric gradient-recalled echo approach. Magn Reson Med 78, 2334-2341, doi:10.1002/mrm.26602 (2017).
- Khrapitchev, A. A., Newling, B. & Balcom, B. J. Sectoral sampling in centric-scan SPRITE magnetic resonance imaging. J Magn Reson 178, 288-296, doi:10.1016/j.jmr.2005.10.006 (2006).
- Ronneberger, O., Fischer, P. & Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. ArXiv abs/1505.04597 (2015).
Figures
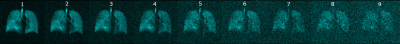
Figure 1. Pseudo wash-out 129Xe lung images obtained in healthy-volunteer.
Depicts 8 images (1-8) generated using the proposed averaging pattern and one originally acquired image (9). SNR of the first image (9 k-space averages) was 20.0 and SNR of the last image was 6.6.
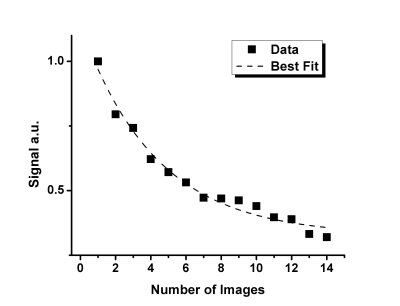
Figure 2. Signal intensity dependence of the 14 human lung k-spaces following the averaging pattern, with the exponential fitting represented by the dashed line.
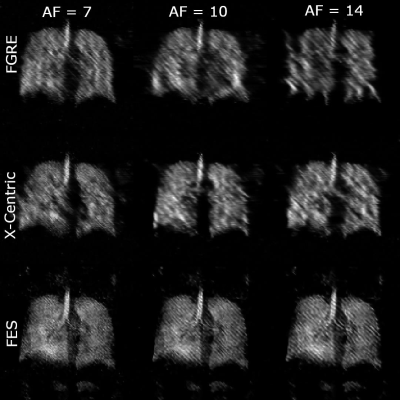
Figure 3. Lung images for all three acceleration factors (7, 10, 14) and three sampling schemes (FGRE, x-Centric, FE-Sectoral); SNR values were > 21 for FGRE, x-Centric, and FES. Frequency-Encoding Sectoral appears to suit this method much better than the other two pulse sequences.
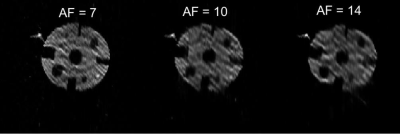
Figure 4. Reconstructions of the undersampled FGRE phantom images using three different acceleration factors (7, 10, 14), showing a higher degree of artefacts at higher AF.
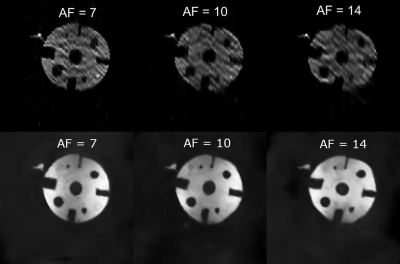
Figure 5. Phantom reconstructions from Figure 4 before (top row) and after (bottom row) applying the Deep-Learning artefact removal network.