0949
IMPULSED model-based brain tumor microstructural parameter estimation with deep neural network1Xiamen University, Xiamen, China, 2Zhongshan Hospital Afflicated to Xiamen University, Xiamen, China, 3MSC Clinical & Technical Solutions, Philips Healthcare, Shenzhen, China
Synopsis
This study assesses the feasibility of training a convolutional neural network (CNN) for IMPULSED (imaging microstructural parameters using limited spectrally edited diffusion) model fitting to diffusion-weighted (DW) data and evaluates its performance on a brain tumor (poorly differentiated adenocarcinoma) patient data directly acquired from clinical MR scanner. Comparisons were made with the results calculated from the non-linear least squares (NLLS) algorithm. More accurate and robust results were obtained by our CNN method, with processing speed several orders of magnitude faster than the reference method (from 5 min to 1 s).
Introduction
Physiological properties of cellular scale in tumor microenvironment have been closely related to therapeutic response and prognosis. Cell size is a fundamental feature of living tissues and plays a vital role during proliferation, metabolism, and cell death. Quantitative analysis of microstructural parameters such as cell size is typically performed with biopsies. Despite of extensive imaging techniques to investigate cellularity parameters in experimental or preclinical studies, most of the methodologies can hardly translate to real clinical settings. Recent work proposed an approach to characterize mean cell size in human cancer patient by using diffusion-weighted (DW) magnetic resonance imaging with IMPULSED (imaging microstructural parameters using limited spectrally edited diffusion) model, representing a general feasible way to manage tissue parameter interpretation noninvasively.1,2 In terms of biophysical model fitting, although traditional non-linear least squares (NLLS) fitting method is capable of estimating parameters with considerable accuracy, it is relatively slow as a result of complicated mathematical modeling and verbose recursive iterations, and the outcomes are heavily depended on weakly informative priors. Recently, there has been a renewed interest in using artificial neural networks for parameter fitting in studies such as intra-voxel incoherent motion,3 ,4 and chemical exchange saturation transfer.5,6 Our current study aims to assess the feasibility of applying convolutional neural network (CNN) for IMPULSED model fitting to DW images and evaluate its performance on clinically acquired patient data.Methods
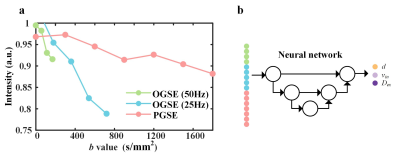
Training samples generation: As shown in Figure 1a, training data were synthesized5 using the MATI (microstructural analysis of tissues by imaging toolbox)1 with added Rician noise. DW signals were generated with various mean cell size (d), intracellular volume fraction (vin), and extracellular diffusion coefficient (Dex) based on the IMPULSED model. The ranges of d, vin, and Dex were selected as 0 - 30 μm, 0 - 60 %, and 0 - 3 μm2/ms, respectively.Reconstruction: We implemented the CNN as shown in Figure 1b. At the training stage, the data were randomly split to three parts: training (70%), validation (15%), and test (15%). Adam optimizer was used with an initial learning rate of 0.0001 and a weight decay factor of 0.5 for every 10,000 iterations. The network was trained with a batch size of 8 for 100,000 iterations in total. At testing stage, the experimental data were fed into the pre-trained U-Net to obtain multiple parametric maps. Then relative apparent diffusion coefficient (ADC) change map was calculated as (ADC25Hz-ADCPGSE)/ADCPGSE.
Experiments: One patient (Male, age = 62) under an IRB-approved was recruited and imaged on a 3.0T MR scanner (Ingenia CX, Philips Healthcare) equipped with a dedicated head coil of 32-channel, a maximum gradient strength of 80 mT/m and maximum gradient slew rate of 200 mT/m/s. The experiment was performed with our homemade IMPULSED sequence (TE/TR, 106 ms/3000 ms; FOV, 220×220 mm2).
Results
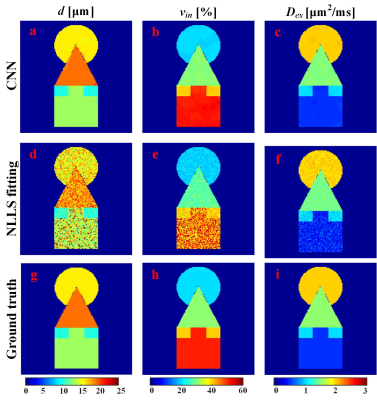
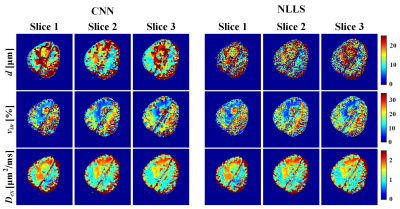
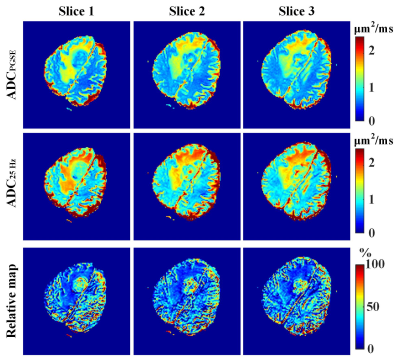
To test the performance of the trained neural network, we generate DW signals pixel by pixel based on the ground-truth maps shown in Figure 2g-i. NLLS fitting was also performed for comparison. Quantitative results in Figure 2a-f show that CNN can yield better fidelity and are more robust to noise compared with the NLLS fitting. It should be noted that CNN takes less than 1 s for the parametric mapping task on our server, while NLLS fitting requires around 5 min under the same condition (Inter E5-2620 with 128 G memory). The same CNN was applied to quantify DW data obtained from the clinic, and the results are shown in Figure 3. In the parametric maps computed by CNN, the tissues appear more homogeneous, whereas much noisy maps are observed with NLLS approach, especially for cell size estimation. The relative ADC change maps are shown in Figure 4, and the tumor margin can be clearly visible using our approach, which appears much obscure in the traditional ADC maps.Conclusion
This work illustrates the feasibility of training a CNN to estimate microstructural parameters. The trained CNN can reliably and accurately predict multiple microstructural properties and speed up the whole process by a large margin.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China under grant numbers 11775184, 82071913, and 82102021, in part by Science and Technology Project of Fujian Province 2019Y0001 and in part by the China Postdoctoral Science Foundation under grant 2020M671947.References
[1] Xu J, Jiang X, Li H, et al. Magnetic resonance imaging of mean cell size in human breast tumors. Magn. Reson. Med., 2020, 83(6): 2002-2014.
[2] Xu J, Jiang X, Devan SP, et al. MRI-cytometry: Mapping nonparametric cell size distributions using diffusion MRI, Magn. Reson. Med., 2021, 85(2): 748-761.
[3] Kaandorp M P T, Barbieri S, Klaassen R, et al. Improved unsupervised physics‐informed deep learning for intravoxel incoherent motion modeling and evaluation in pancreatic cancer patients. Magn. Reson. Med., 2021, 86(4): 2250-2265.
[4] Barbieri S, Gurney‐Champion O J, Klaassen R, et al. Deep learning how to fit an intravoxel incoherent motion model to diffusion‐weighted MRI. Magn. Reson. Med., 2020, 83(1): 312-321.
[5] Chen L, Schär M, Chan K W Y, et al. In vivo imaging of phosphocreatine with artificial neural networks. Nat. Commun., 2020, 11, 1072.
[6] Guo C, Wu J, Rosenberg J T, et al. Fast chemical exchange saturation transfer imaging based on PROPELLER acquisition and deep neural network reconstruction. Magn. Reson. Med., 2020, 84(6): 3192-3205.
Figures