0948
A Cartilage-Specific Loss Function Improves Image Reconstruction Performance in Multiple Tissues of Clinical Interest
Aniket Tolpadi1,2, Francesco Calivà1, Misung Han1, Emma Bahroos1, Peder Larson1, Sharmila Majumdar1, and Valentina Pedoia1
1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Bioengineering, University of California, Berkeley, Berkeley, CA, United States
1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Bioengineering, University of California, Berkeley, Berkeley, CA, United States
Synopsis
Most MRI image reconstruction algorithms are optimized for full-volume performance rather than specific tissues. Using a KIKI-Net style architecture and multi-component loss function in image space and k-space as baseline, we find a cartilage-specific loss function improves reconstruction performance at R=4 and R=8 in both cartilage and menisci. Thus, it may be possible to improve clinical utility of reconstruction pipelines across tissues of heightened clinical interest using a simple loss function weighting. Furthermore, full-volume standard reconstruction metrics worsened at R=4 and R=8 while tissue-specific metrics improved, calling into question whether these metrics are best for assessing reconstruction pipeline clinical utility.
Introduction
For musculoskeletal imaging, MRI is a premier option, offering high-resolution images with exquisite soft tissue contrast1. A major drawback, however, is long acquisition time. Consequently, image reconstruction has been a major recent research focus, with deep learning, compressed sensing, and model-based approaches among those under development to accelerate acquisition2-6. However, most published approaches are optimized for entire imaging volumes rather than specific tissues of interest. While not necessarily a drawback, musculoskeletal imaging often necessitates strong image quality most so in a specific tissue such as cartilage7. Moreover, most reconstruction algorithms are assessed with metrics like structural similarity index (SSIM)8 that are agnostic to tissues of interest and may not accurately capture a model’s clinical utility9. As such, optimization of reconstruction algorithms for specific tissues remains an open question, and the limitations of standard image reconstruction metrics is worth investigating.Methods
Image AcquisitionFor 3D-Fast-Spin-Echo fat suppressed CUBE images acquired at a UCSF GE Signa 3T MRI scanner, an in-house pipeline was developed that leveraged GE Orchestra 1.10 and other postprocessing tools to reconstruct images from raw scanner data, allowing multicoil k-space, pre-processed coil-combined images, post-processed coil-combined images, and other intermediate files from the image processing pipeline to be stored. Acquisition parameters were as follows: FOV=15cm2; acquisition matrix=256×256×200; ±62.5kHz readout bandwidth; TR=1002ms; TE=29ms; ARC acceleration by a factor of 410. Images are zero-filled in k-space to obtain final 512×512×200 resolution. Scans from 62 patients were split 38/12/12 into training, validation, and test.
Undersampling and Pre-Processing
3D multicoil k-space was undersampled in ky-kz with a center-weighted Poisson pattern while fully sampling the 5% central square in k-space, projecting the pattern along kx. Both undersampled and fully-sampled k-space were 1D inverse Fourier transformed along the slice direction, yielding undersampled and corresponding ground truth kx-ky-z 2D k-space for each coil, along with associated multicoil images. Root sum of squares coil combination of fully sampled coil images was used to calculate ground truth coil-combined images. A 5-class 3D V-Net pipeline obtained segmentations for 4 knee cartilage compartments and the menisci11.
Training
A KIKI-Net12 inspired architecture was designed to take 2D undersampled multicoil images as input, predict fully sampled multicoil images, and do root sum of squares coil combination, yielding a coil-combined prediction (Fig. 1). A 4-component loss function was used to train baseline networks at R=4 and R=8: (1) multicoil image space L1 loss; (2) multicoil k-space L1 data consistency loss; (3) coil-combined image space L1 loss; (4) 1 - coil-combined SSIM. In addition, separate networks were trained with an additional loss component: coil-combined L1 loss in cartilage. Training was done for 20 epochs, with loss function weightings and learning rate being optimized in a hyperparameter search. Standard reconstruction metrics such as SSIM, as well as tissue-specific metrics like normalized root mean square error (nRMSE) and peak signal-to-noise ratio (PSNR) were used to assess performance13.
Results
Standard reconstruction metrics show that at R=4 and R=8, baseline models perform well in recovering ground truth images, with reasonably high SSIM and low nRMSEs (Fig. 2). At a tissue level, these metrics clearly show addition of the cartilage L1 loss reduced nRMSE and increased PSNR not only within cartilage, but also within menisci.Visualizations of reconstructions at R=4 show both pipelines recover fine details and yield dramatic improvements in image quality over zero-filling (Fig. 3). Moreover, reconstructions show the cartilage L1 loss mitigates spurious signal elevations in menisci present in baseline models while more accurately reconstructing sharp, local signal elevations. While trends were similar at R=8, the cartilage L1 loss also mitigated aliasing artifacts in menisci slightly better than the baseline model (Fig. 4).
Discussion and Conclusions
In tandem with the proposed architecture, a cartilage L1 loss term improved reconstruction performance not only within cartilage, but also within menisci. While the former is interesting and useful, it is unsurprising. However, a cartilage loss term that improves meniscal reconstruction in addition to cartilage indicates adding tissue-specific loss terms can improve reconstruction performance in the multiple clinically crucial tissues, offering a simple means of improving reconstruction performance for clinical settings worthy of exploration for all reconstruction algorithms. Also noteworthy is that at R=4 and R=8, full-slice SSIM, nRMSE, and PSNR worsened despite improvements in cartilage and menisci reconstructions. This indicates that, just as pulse sequence techniques such as fat suppression improve image quality in one tissue at the expense of another, reconstruction pipelines too can and perhaps should be optimized for tissue-specific performance. Another option may be training multiple tissue-specific pipelines and aggregating predictions to obtain improved full-volume reconstructions. Beyond these possibilities, that full-volume metrics worsened as cartilage and meniscal reconstructions improved raises the question of whether these standard metrics are optimal to evaluate clinical utility of reconstruction pipelines, seeing that the most clinically useful pipeline is likely one with very strong cartilage and menisci reconstructions.This work elucidates the potential of tissue-specific losses to improve clinical utility of reconstruction models. To further investigate this, future work will include extension of this approach to other anatomies. Lastly, particularly for 3D sequences, finding innovative ways to exploit all dimensions of the acquisition in training while managing computational constraints is another avenue of future exploration.
Acknowledgements
We would like to acknowledge Bruno Astuto Arouche Nunes for developing and running the multiclass knee cartilage and menisci segmentation pipeline. We also acknowledge Pablo Damasceno for assistance in parallelizing image reconstructions and saving results offline to speed up eventual training time. Additionally, we also acknowledge Andrew Leynes for developing a script that was adapted to automatically transfer raw scanner data from the 3T GE Sigma Scanners to local file systems. Moreover, we acknowledge Rutwik Shah, Upasana Bhardwaj, and Thomas Link for providing expert assessments regarding the quality of images reconstructed from the in-house pipeline, and Peder Larson for assistance in selecting undersampling patterns to use for training algorithms. Finally, we also would like to acknowledge our funding sources NIH UH3AR076724, NIH R01AR078762, and NIH R01AR069006.References
- Roos, EM, Arden, NK. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016;12:92-101.
- Sriram A, Zbontar J, Murrell T et al. GrappaNet: Combining Parallel Imaging with Deep Learning for Multi-Coil MRI Reconstruction. arXiv Preprint. 2020.
- Dwork N, O’Connor D, Baron CA et al. Utilizing the wavelet transform’s structure in compressed sensing. Signal Image Video Process. 2021;15:1407-14.
- Schlemper J, Oksuz I, Clough J et al. dAUTOMAP: decomposing AUTOMAP to achieve scalability and enhance performance. arXiv Preprint. 2019.
- Yaman B, Hosseini SAH, Akcakaya M. Zero-Shot Self-Supervised Learning for MRI Reconstruction. arXiv Preprint. 2021.
- Aggarwal HK, Jacob M. J-MoDL: Joint Model-Based Deep Learning for Optimized Sampling and Reconstruction. In IEEE Journal of Selected Topics in Signal Processing. 2020;14(6):1151-62.
- Crema MD, Roemer FW, Marra MD et al. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. Radiographics. 2011;31(1). https://doi.org/10.1148/rg.311105084
- Wang Z, Bovik AC, Sheikh HR et al. Image Quality Assessment: From Error Visibility to Structural Similarity. In IEEE Transactions on Image Processing. 2004;13(4):600-12.
- Knoll F, Murrell T, Sriram A et al., Advancing machine learning for MR image reconstruction with an open competition: Overview of the 2019 fastmri challenge. Magn Reson Med. 2020;84(6):3054-70.
- Brau AC, Beatty PJ, Skare S et al. Comparison of reconstruction accuracy and efficiency among autocalibrating data‐driven parallel imaging methods. Magn Reson Med. 2008;59(2):382-395.
- Nunes BAA, Flament I, Shah R et al. MRI-based multi-task deep learning for cartilage lesion severity staging in knee osteoarthritis. Osteoarthr Cartil. 2019;27(1):S398-99. https://doi.org/10.1016/j.joca.2019.02.399
- Eo T, Jun Y, Kim T et al. KIKI-net: cross-domain convolutional neural networks for reconstructing undersampled magnetic resonance images. Magn Reson Med. 2018;80(5):2188-201.
- Pal A, Rathi Y. A review of deep learning methods for MRI reconstruction. arXiv Preprint. 2021.
Figures
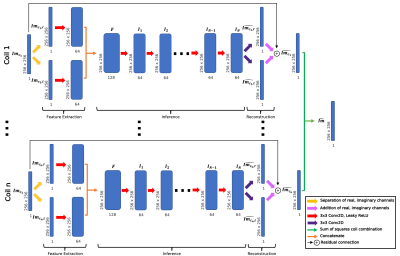
Fig. 1: KIKI-Net inspired architecture predicts coil-combined images from undersampled multicoil image-space inputs. Undersampled coil images were fed through feature extractors for real and imaginary channels, inference convolutions, and a reconstruction convolution, and subsequently sum of squares combined to yield single-coil predictions. Weights were shared across coils, and N=10 inference layers used. Baseline networks were trained with a multi-component loss: multicoil image space L1, multicoil k-space L1, coil-combined L1, coil-combined SSIM.
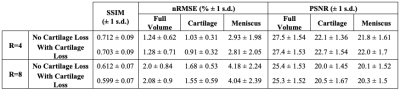
Fig. 2: Model performance metrics evaluated across the test set. Reconstructions within cartilage and menisci show slight to substantially lower errors and higher PSNR at expense of slight drops in full-slice performance with cartilage-specific loss usage. Tissue-specific losses can thus improve reconstruction performance in multiple clinically relevant tissues. Lower SSIM compared to other published models is likely in part due to the challenges associated with reconstructing such a high-resolution, fat suppressed sequence.
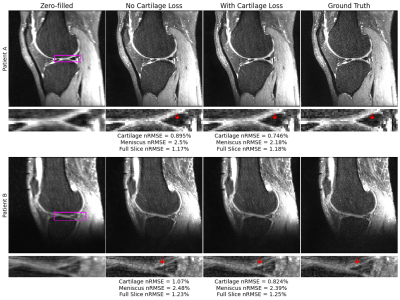
Fig. 3: Zero-filled initializations, ground truth, and reconstructions from pipelines with and without the cartilage-specific loss at R=4. In both patients, nRMSEs show improvement in cartilage and menisci reconstructions with use of a cartilage-specific loss. In patient A, standard reconstruction reveals a slight, spurious elevation in posterior meniscal horn signal that is less apparent with cartilage-specific loss. Similarly in patient B, a sharp signal elevation in lateral femoral cartilage is better reconstructed with cartilage-specific loss.
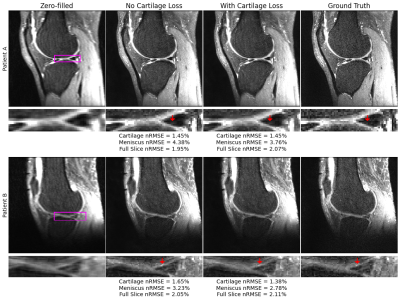
Fig. 4: Reconstructions at R=8 show similar or improved nRMSE in cartilage and menisci with cartilage-specific loss use. In patient A, an aliasing artifact in both reconstructions is better managed, although not eliminated, in the posterior meniscal horn with use of cartilage specific loss. In patient B, similar to the R=4 pipeline, the sharp local elevation in lateral femoral cartilage signal is better reproduced with a cartilage-specific loss, indicating improvements in both cartilage and menisci reconstructions with the tissue-specific loss.
DOI: https://doi.org/10.58530/2022/0948