0935
Peri-tumoural spatial distribution of lipid composition and tubule formation in breast cancer1Institute of Medical Sciences, University of Aberdeen, Aberdeen, United Kingdom, 2Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, Netherlands, 3Pathology Department, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 4Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom
Synopsis
The spatial distribution of lipid composition in breast has a major role in breast cancer prevention, with deregulation of lipid metabolism identified in BRCA1/2 genetic mutation carriers. Neoplastic tubule formation, a key component of grading score, is directly associated with cellularity, with significant implications on prognosis. Lipid composition measurement through biochemical extraction is invasive, while conventional spectroscopic imaging demands long acquisition time. Novel chemical shift-encoded imaging (CSEI) allows lipid composition mapping of the whole breast in a clinically acceptable timeframe. We set out to examine the relationship between peri-tumoural lipid composition and tubule formation using CSEI in breast tumours.
Introduction
Breast cancer is a major and expanding health challenge, and the deregulation of lipid composition in the breast has been suggested as a risk factor1,2. Correlation spectroscopy has shown abnormal lipid metabolism in the breast of BRCA1/2 genetic mutation carriers3, however the acquisition is prolonged and limited to a single spatial location (single voxel). Recent development in gradient-echo based chemical shift-encoded imaging (CSEI) allows rapid lipid composition mapping, utilising the known resonant frequencies of lipids with a theoretical model on specific abundance and structure of triglycerides4-6. We therefore conducted a cross-sectional study to examine the relationship between peri-tumoural lipid composition and neoplastic tubule formation, an indicator for tumour differentiation from normal breast ducts and lobules, in freshly excised breast tumour.Methods
Twenty patients (9 Score 2 and 11 Score 3 in tubule formation) with invasive breast carcinoma participated in the study. Patients undergoing breast conservation surgery, with no previous malignancies, chemotherapy or radiotherapy prior to surgery were eligible. The study was approved by the North West – Greater Manchester East Research Ethics Committee (Identifier: 16/NW/0221), and signed written informed consents were obtained from all participants (Figure 1).Specimen Preparation
Upon tumour excision, the tissue specimen was submerged in 10% formalin to prevent tissue degradation and immobilised by a custom-built holding harness. The tissue specimen was immediately transported from operating theatre to Aberdeen Biomedical Imaging Centre in a sealed container for lipid composition mapping.
MRI Acquisition
The MRI data for all 20 excised breast tissue specimens were acquired at room temperature on a 3 T whole body clinical MRI scanner (Achieva TX, Philips Healthcare, Best, Netherlands) using a 32-channel receiver coil for high sensitivity detection and a body coil for uniform transmission. Anatomical images were acquired using standard T1-weighted 3D sequence7 with repetition time (TR) of 5.7 ms, echo time (TE) of 2.9 ms, field of view of 141 × 141 mm2, matrix size of 256 × 256, 28 slices, slice thickness of 1.1 mm, acceleration factor of 1.5. Lipid composition images were acquired using CSEI with an isotropic resolution of 2.2 mm, first TE of 1.14 ms, echo spacing of 1.14 ms, 16 echoes, TR of 20 ms, flip angle of 6° and 9 signal averages4-6.
Data Processing
Regions of interests (ROI) were drawn on CSEI magnitude images to define the adipose tissue boundary. A theoretical model4 was used to map the number of double bonds in triglyceride molecules from the magnitude data on a pixel-by-pixel basis, from which the lipid components, including mean monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA)4,5 were derived. Fat fraction, defined as the ratio between fat and the sum of fat and water, was also computed. The following inclusion criteria were set: (1) fat fraction must be greater than or equal to 60% and (2) MUFA/PUFA/SFA must not exceed beyond the range between 0% and 100%, thus non-adipose tissue and voxels with low SNR did not contribute to the outliers of the analysis. The spatial distribution (skewness, entropy and kurtosis) was computed based on histogram analysis for each lipid component.
Statistical Analysis
All statistical analysis was performed in the SPSS software (Release 23.0, SPSS Inc., Chicago, IL, USA). Shapiro-Wilk test for normality was performed on all the collected data. Student’s t or Mann Whitney U tests were performed to assess difference in lipid components between Scores 2 and 3. The correspondence between lipid components against proliferative activity marker Ki-67 was examined using Spearman’s rank correlation test. A p value < 0.05 was considered statistically significant.
Results
For MUFA, there was a significantly lower mean (0.38 ± 0.02, p = 0.012, Figure 2a, Table 1), higher skewness (-1.35 ± 0.75, p = 0.012, Figure 2b), higher entropy (4.94 ± 0.24, p = 0.022, Figure 2c), and a significantly lower kurtosis (5.44 (4.08 – 7.21), p = 0.038, Figure 2d) in Score 3. For MUFA against Ki-67, there were significant correlations in mean (ρ = -0.54, p = 0.014, Figure 3a, Table 1), skewness (ρ = 0.60, p = 0.005, Figure 3b), entropy (ρ = 0.67, p = 0.001, Figure 3c), but not in kurtosis (Figure 3d). For SFA, there was a significantly higher mean (0.51 ± 0.04, p = 0.033, Figure 4a, Table 1) and lower skewness (-0.39 ± 0.37, p = 0.011, Figure 4b) in Score 3, but not in entropy or kurtosis. For SFA against Ki-67, there was significant correlation in mean (ρ = 0.59, p = 0.006, Figure 4c, Table 1), but not in skewness (Figure 4d). For PUFA, there were no significant differences in mean, skewness, entropy or kurtosis between the groups (Table 1).Discussion
Lipid deregulation in the peri-tumoural region was associated with tumour proliferation. Neoplastic tubule formation, an indicator of cellular differentiation, is associated with structural changes in the tumour and may impact on peri-tumoural lipid composition due to the consumption of specific types of lipids for membrane synthesis.Conclusion
There was an association between peri-tumoural spatial distribution of lipid composition with tumour cellular differentiation. Lipid composition imaging might have potential in non-invasive quantitative assessment of patients with breast cancer for treatment planning and monitoring.Acknowledgements
The authors would like to thank Dr Matthew Clemence (Philips Healthcare Clinical Science, UK) for clinical scientist support, Ms Bolanle Brikinns for patient recruitment support and Ms Dawn Younie for logistic support. This project was funded by NHS Grampian Endowment Research Fund. Sai Man Cheung’s PhD study was jointly supported by Elphinstone scholarship, Roland Sutton Academic Trust and John Mallard scholarship and is currently funded by Cancer Research UK. Nicholas Senn’s PhD study was supported by BBSRC EASTBIO scholarship.References
1. Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498-1503.
2. Wang YY, Attané C, Milhas D, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):1-20.
3. Ramadan S, Arm J, Silcock J, et al. Lipid and Metabolite Deregulation in the Breast Tissue of Women Carrying BRCA1 and BRCA2 Genetic Mutations. Radiology. 2015;275(3):675-682.
4. Bydder M, Girard O, Hamilton G. Mapping the double bonds in triglycerides. Magn Reson Imaging. 2011;29(8):1041-1046.
5. Peterson P, Månsson S. Simultaneous quantification of fat content and fatty acid composition using MR imaging. Magn Reson Med. 2013;69(3):688-697.
6. Bydder M, Hamilton G, de Rochefort L, et al. Sources of systematic error in proton density fat fraction (PDFF) quantification in the liver evaluated from magnitude images with different numbers of echoes. NMR Biomed. 2018;31(1):e3843–10.
7. Thomassin-Naggara I, Trop I, Lalonde L, David J, Péloquin L, Chopier J. Tips and techniques in breast MRI. Diagnostic and Interventional Imaging. 2012;93(11):828-839.
Figures
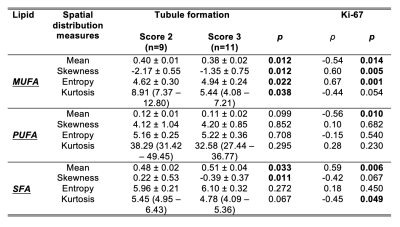
Table 1. Differences in peri-tumoural lipid components and correlations with proliferative activity marker Ki-67.
Peri-tumoural monounsaturated, polyunsaturated and saturated fatty acids (MUFA, PUFA, SFA) mean, skewness, entropy and kurtosis were compared between tubule formation Scores 2 and 3. The Spearman’s rank correlation coefficients (ρ) between lipid components against proliferative activity marker Ki-67 are also shown. Statistical significant differences (p < 0.05) are marked in bold.
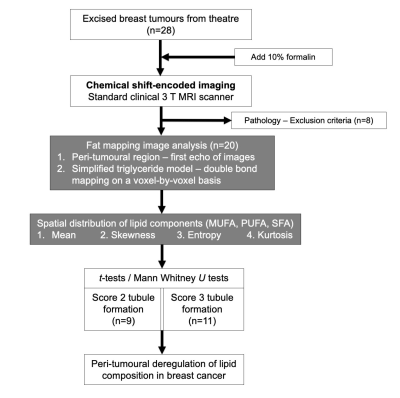
Figure 1. Study design.
A two-group cross-sectional study in a flow chart. Lipid composition maps were acquired using chemical shift-encoded imaging (CSEI) on standard clinical 3 T MRI scanner. Regions of interests were drawn on CSEI images to define the adipose tissue boundary. Fat, water and the number of double bonds in triglyceride molecules were estimated from the CSEI data to compute the metrics of monounsaturated, polyunsaturated and saturated fatty acids (MUFA, PUFA and SFA). Statistical comparison was conducted on lipid components between Scores 2 and 3 tubule formation.
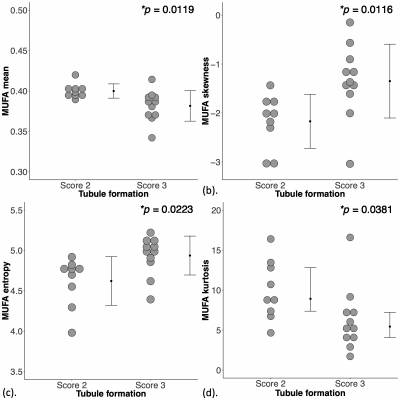
Figure 2. Group differences in monounsaturated fatty acids (MUFA) in peri-tumoural breast adipose tissue.
The difference in MUFA (a) mean, (b) skewness, (c) entropy and (d) kurtosis between breast cancer with Scores 2 and 3 tubule formation are shown in dot plots. Each dot represents the spatial distribution around the breast tumour, and the dots are organised in two columns corresponding to the two groups. The error bar indicates the mean and standard deviation. The t-tests were performed and p value is shown for each plot. Statistical significant p values (< 0.05) are marked by ‘*’.
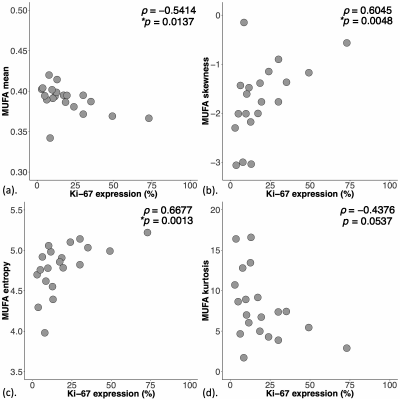
Figure 3. Correlations of peri-tumoural monounsaturated fatty acids (MUFA) with tumour proliferative activity marker Ki-67.
The correlation of MUFA (a) mean, (b) skewness, (c) entropy and (d) kurtosis against tumour proliferative activity marker Ki-67 are shown in scatter plots. The corresponding Spearman’s rank correlation coefficients (rho (ρ) score) and p values are displayed. Statistical significant p values (< 0.05) are marked by ‘*’.
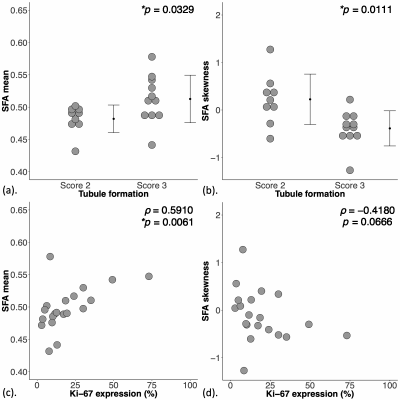
Figure 4. Group difference and correlation of saturated fatty acids (SFA) in peri-tumoural breast adipose tissue.
The difference in SFA (a) mean and (b) skewness are shown in dot plots. Each dot represents a peri-tumoural spatial distribution, and the dots are organised in two columns corresponding to tubule formation Scores. The t-tests were performed and p value is shown. The Spearman’s rank correlation (rho (ρ) score) of SFA (c) mean and (d) skewness against proliferative activity marker Ki-67 are shown in scatter plots. Statistical significant p values (< 0.05) are marked by ‘*’.