0933
Changes in cortical blood flow >1 year after radiation for glioma using arterial spin labelling MRI1Medical Physics and Biomedical Engineering, University College London Hospitals, London, United Kingdom, 2Radiotheraphy, University College London Hospitals, London, United Kingdom, 3Department of Medical Physics and Biomedical Engineerin, University College London, London, United Kingdom, 4Imaging, University College London Hospitals, London, United Kingdom
Synopsis
This work proposes a framework for monitoring brain perfusion after radiotherapy treatment of glioma in relation to the radiotherapy dose.
Introduction
Radiotherapy (RT) plays a vital role in management of brain malignancies but is associated with significant late effects including neurocognitive impairment and stroke [1], [2]. Currently, RT is planned to deliver maximum dose to the tumour while avoiding anatomies such as optic chiasma, cochlea, etc, which are recognised as organs at risk [3]. However, there is an increased interest in investigation of the short and long-term effects of RT on the brain parenchyma that is possible with quantitative MRI methods, such as arterial spin labelling (ASL) [4]. ASL enables quantification of cerebral blood flow (CBF) non-invasively and can be used for clinical follow up surveillance of patients after RT, however, combination of RT plans, corresponding CT image and MRI data can be problematic. In this work, we develop a data framework to enable a retrospective study to determine whether there are any very long-term (i.e. beyond one year after RT) CBF changes outside the treatment target and whether these correlate with delivered dose. Quantifying long term changes in brain parenchyma could help in refining RT planning and monitoring for toxicities more effectively.Methods
Patients: Glioma patients who received high dose RT (54-60Gy) who had also undergone ASL-MRI 12-18 months after completion of RT at our centre were identified from our institutional databases and electronic medical records for inclusion.Imaging: ASL was performed using pseudo-continuous labelling (pCASL) [5] and 2D EPI readout on a 3T MRI (Achieva or Ingenia, Philips Medical Systems, Best, The Netherlands). Sequence parameters were: labelling duration 1.8s, post labelling delay 2s, background and fat suppression. Additional proton density image was also required for quantification.
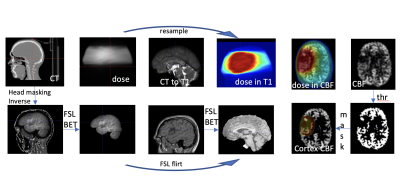
Post-processing: Individual ASL volumes were first motion corrected using rigid registration (DTI-TK package) and quantified using simplified Buxton model. Cortex voxels were defined within CBF maps using a threshold (30-120ml/100g/min). RT dose data and planning CT images were exported from Eclipse (Varian Medical Systems, Palo Alto, USA), converted, and aligned to pre-contrast high resolution T1-weighted image (FSL package). Next, dose maps were down-sampled to CBF map resolution (figure 1). Finally, RT dose was divided into six bins, and CBF data were normalised to the maximum value from the lowest dose bin and obtained for all subjects.
Results
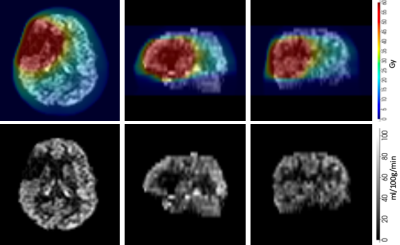
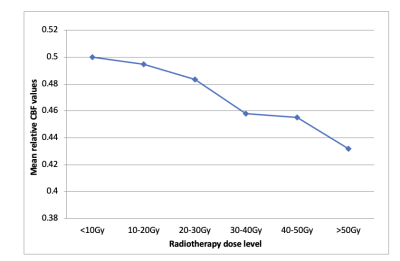
Fifty four patients were identified as suitable for inclusion. Approximately 90 percent had pathologically confirmed high grade glioma (WHO Grade III/IV) and received RT doses of 59.4-60Gy in 1.8-2Gy daily fractions, with most of the remainder receiving 54Gy in 1.8Gy daily fractions for WHO Grade II tumours. Testing of the function of the proposed framework outlined above was undertaken on a cohort of five randomly chosen patients. An example of a CBF map with and without dose overlay is shown in figure 2. ASL perfusion data from these five patients shows a dose-dependent reduction in cortical perfusion outside the target volume, with a reduction in mean relative CBF values from 0.5 at the lowest radiation doses (<10Gy) to 0.43 at doses >50Gy (figure 3).Conclusions
A data framework for combining ASL perfusion data with RT dosimetric data has been developed. Early data indicate that reductions in grey matter CBF persist outside the target volume over one year after RT for glioma, and are dose dependent. This has implications for radiotherapy planning and post-treatment toxicity surveillance and potentially its management. Further analysis of the full patient cohort will be undertaken and available for presentation.Acknowledgements
No acknowledgement found.References
[1] Brown PD et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J Clin Oncol. 2003 Jul 1;21(13):2519-24.
[2] Black DF et al. SMART: stroke-like migraine attacks after radiation therapy. Cephalalgia. 2006 Sep;26(9):1137-42.
[3] Scoccianti et a.l Organs at risk in the brain and their dose-constraints in adults and in children: A radiation oncologist’s guide for delineation in everyday practiceRadiotherapy and Oncology 114 (2015) 230–238
[4] Petr J et al. Early and late effects of radiochemotherapy on cerebral blood flow in glioblastoma patients measured with non-invasive perfusion MRI. Radiother Oncol. 2016 Jan;118(1):24-8
[5] D. C. Alsop et al., “Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia,” Magn. Reson. Med., vol. 73, no. 1, pp. 102–116, 2015.