0931
Integrative Hyperpolarized 13C Metabolic and Standard-of-Care Abdominopelvic Staging/Restaging MRI for Patients with Advanced Solid Tumors1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Harnessing unique metabolic insight from hyperpolarized (HP) 13C MRI, this study describes an integrated approach toward routine cancer staging and restaging using combined HP 13C plus standard of care (SOC) MRI with commercially-available 13C and 1H array coils and a time-efficient adaptive protocol. A series of patients with osseous, liver, nodal metastases, and locally advanced primary tumors suggested HP 13C not only detected high metabolic activity in progressive tumors, but it also revealed inter- and intratumoral metabolic heterogeneity. Results indicated HP 13C-MRI can greatly complement SOC-MRI in assessing therapeutic response and guiding clinical decisions.
Purpose
Imaging is a centerpiece in the world of clinical oncology. Staging and restaging scans are routinely ordered to guide standard-of-care (SOC) management and treatment decisions via characterizing the extent of the disease (i.e. staging) and monitoring treatment response.Although conventional imaging is the mainstay of cancer staging, size-based response criteria often prove ineffective for osseous metastases1, and patients with bone-only malignancies such as prostate and breast cancers are often precluded from clinical trials for lack of measurable disease2. There is mounting evidence that hyperpolarized (HP) 13C-pyruvate metabolic MRI detects early response/non-response to therapy in otherwise non-measurable disease, and that metabolic-based changes can precede traditional RECIST-based measurements3-5.
Harnessing HP 13C’s unique metabolic insight, this study proposes to integrate HP+SOC staging/restaging MRI using commercially-available ergonomic array coils and a time-efficient adaptive protocol, evaluated via a case series characterizing advanced malignancies involving bone, visceral, soft tissue/nodal and primary/recurrent diseases.
Methods
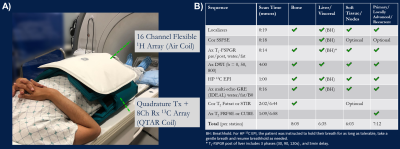
Hardware and Coils: This study used a 3T clinical magnet (MR750, GE Healthcare). A flexible 13C coil with quadrature volume transmit and 8-channel array receivers (“QTAR”, Clinical MR Solutions), wrapped around the torso, provided up to RLxAPxSI=33x28x22cm volumetric coverage of the abdomen or pelvis (Fig.1A). A 16-channel blanket-style 1H array (“Air Coil”, GE Healthcare), whose linked resonators replaced traditional copper-based coil loops, minimized 1H-13C coupling and offered high-quality SOC imaging commensurate to the 13C volume. Based on the focus of the exam and tissues to characterize, Air Coil could be positioned either anteriorly over (e.g. liver mets), or posteriorly underneath (e.g.iliac involvement) the QTAR.Protocol Design: Sequences for the integrated HP and SOC-MR of osseous, visceral, soft tissue and primary/recurrent tumors are outlined in Figure 1B, having flexibilities to “mix and match” when involving multiple tissue types. Principally, these comprise HP 13C EPI, T1-weighted pre and post, T2-weighted with or without fatsat, DWI, and multi-echo GRE (IDEAL). In conjunction with Air Coil, SOC imaging was accelerated using GRAPPA6. Surface coil intensity profile was corrected using built-in PURE or SCIC7.
Hyperpolarized-13C MR Exam: GMP [1-13C]pyruvate was polarized in a 5T research polarizer (Spinlab, GE Healthcare) for 2.5-3 hours. Dissolution yielded 235±12 mM pyruvate solution with 33±3% polarization, 7.7±0.1pH, radical concentration 1.7±0.3 μM, and temperature 32±1°C. The pharmaceutical release and injection followed FDA and IND-approved protocols8. EPI acquisition used 4.5cc spatial and 3s temporal resolutions.
Results and Discussions
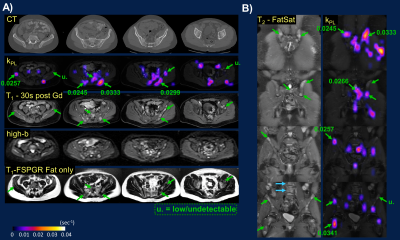
The integrative 13C/SOC protocol was routinely performed within 1-1.5 hours, inclusive of patient positioning, prescription and 13C frequency/power calibrations. Key contributing factors to the quick turnaround time were optimized protocol with parallel imaging, proficiency of researchers and technologists, and teamwork. Nominal scan time at each station was under 10 minutes. The 13C portion could be further expedited using automatic prescription and calibration techniques9. The IDEAL imaging created a B0 field map with the same shim values as 13C EPI, which was used to assess B0 field homogeneity and could be used to correct for geometric distortion.Flexible 13C QTAR arrays not only improved ergonomics compared to a prior approach using a rigid paddle receiver arrays10, but also provided greater spatial coverage that simplified lesion targeting and coil placement during patient setup. Cross-sectionally, QTAR was able to accommodate a large range of pelvic sizes. Longitudinally, example coverages included iliac crest/L5 to femoral neck (Fig.2B), common iliac to inguinal nodes (Fig.3), or entire upper abdomen (Fig.4). Good spatial coverage is essential to characterize inter- and intratumoral kPL (HP biomarker of pyruvate-to-lactate conversion11) heterogeneity (Figs.2&3) that reflects heterogeneous metabolic activity and response to therapies, and may thus guide subsequent management decisions.
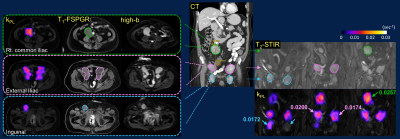
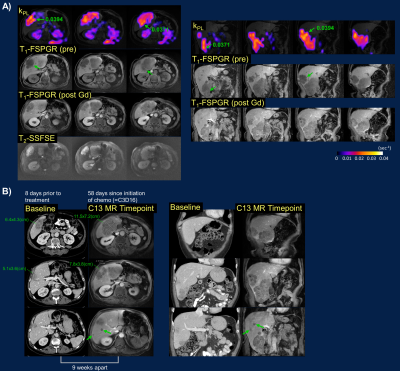
Both 13C&1H MRI detected extensive pelvic bone marrow involvement not conspicuous on contemporaneous CT scan, the latter mostly sensitive to sclerotic changes (Fig.2A)12,13. kPL heterogeneity among these osseous lesions (ranging from undetectable to moderate-high) and within bulky adenopathy (Fig.3) was consistent with patients’ clinically stable or mildly progressive status. Conversely, the universally high kPL over the liver metastases (Fig.4) reflected rapid progression on chemotherapy.
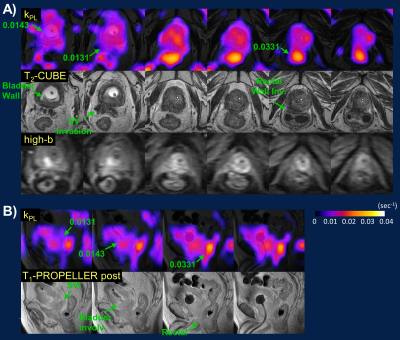
In a patient with clinically stable, locally-advanced prostate tumor (Fig.5), low-moderate kPL correlated with bladder neck and seminal vesicle invasions on T2 and DWI. Interestingly, the rectal wall invasion showed high kPL, possibly reflecting contouring during prior radiation therapy to avoid bowel toxicity. These findings underscore the improved (at a minimum, noninferior) sensitivity of MRI vs CT/bone scan14,15, and the value of a dual metabolic-anatomic approach to cancer staging/restaging.
The bore length and surface coil coverage still limits the 13C portion to either the abdomen or pelvis. The Air Coil can be easily repositioned after pyruvate injection for a clinical abdomen+pelvis order. Alternatively, the body coil provides global coverage with reasonable quality. B1+ variabilities due to vest 13C transmitter conformation (Fig.1A) could be overcome in the future with a birdcage volumetric coil.
Although the proposed 13C+1H array configuration and imaging protocols are validated primarily using a case series of advanced prostate tumors, this general approach would be easily adaptable to staging/restaging advanced solid tumors involving the abdomen/pelvis.
Conclusions
Using commercially available ergonomic 13C+1H MR arrays and a simple, time-efficient yet flexible protocol, this study established a straightforward approach toward integrative metabolic and SOC MR for routine oncologic staging and restaging of advanced solid tumors.Acknowledgements
This work was supported by grants from the NIH (R01CA256740, U01EB026412, R01CA238379, and P41EB013598). We would like to thank Kiersten Cheung, Heather Daniel, Francesca De Las Alas, Romelyn Delos Santos, Evelyn Escobar, Daniel Gebrezgiabhier, Jasmine Hu, Dr. Yaewon Kim, Dr. Philip Lee, Kimberly Okamoto, and Dr. Andrew Riselli for their help with the patient studies.References
[1] Eisenhauer EA et al., New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European journal of cancer 2009; 45(2), 228-247.
[2] Scher HI et al., Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016; 34(12), 1402.
[3] Aggarwal R et al., Hyperpolarized 1-[13C]-pyruvate magnetic resonance imaging detects an early metabolic response to androgen ablation therapy in prostate cancer. Eur. Urol. 2017; 72(6), 1028.
[4] de Kouchkovsky I et al., Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Immune Checkpoint Inhibitor Therapy in Prostate Cancer. Eur. Urol. 2021; https://doi.org/10.1016/j.eururo.2021.10.015.
[5] Woitek R et al., Hyperpolarized 13C MRI of tumor metabolism demonstrates early metabolic response to neoadjuvant chemotherapy in breast cancer. Radiology: Imaging Cancer. 2020; 2(4), e200017.
[6] Griswold MA et al., Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47(6), 1202-1210.[7] Vovk U et al., A review of methods for correction of intensity inhomogeneity in MRI. IEEE Trans Med Imaging 2007; 26(3), 405-421.
[8] Park I et al., Development of methods and feasibility of using hyperpolarized carbon‐13 imaging data for evaluating brain metabolism in patient studies; Magn Reson Med 2018; 80(3), 864-873
[9] Tang S et al., A regional bolus tracking and real‐time B1 calibration method for hyperpolarized 13C MRI. Magn Reson Med 2019; 81(2), 839-851.
[10] Tang S et al., Metabolic imaging with hyperpolarized 13C pyruvate magnetic resonance imaging in patients with renal tumors—Initial experience. Cancer 2021; https://doi.org/10.1002/cncr.33554.
[11] Larson PEZ et al., Investigation of analysis methods for hyperpolarized 13C‐pyruvate metabolic MRI in prostate cancer patients. NMR Biomed 2018; 31(11), e3997.
[12] Rosenthal DI et al., Radiologic diagnosis of bone metastases. Cancer: Interdisciplinary International Journal of the American Cancer Society 1997; 80(S8), 1595-1607.
[13] Yang HL et al., Diagnosis of bone metastases: a meta-analysis comparing 18 FDG PET, CT, MRI and bone scintigraphy. European radiology 2011; 21(12), 2604-2617.
[14] Van Nieuwenhove S et al., Whole‐body magnetic resonance imaging for prostate cancer assessment: Current status and future directions. J. Magn. Reson. Imaging 2020; https://doi.org/10.1002/jmri.27485
[15] Shen G et al., Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal radiology 2014; 43(11), 1503-1513.
Figures