0927
Hyperpolarized 13C Pyruvate MR-Assisted Longitudinal Risk Stratification in Prostate Cancer Patient – From Surveillance to Surgery1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Serial hyperpolarized 13C+mpMRI and paired metabolic image-guided biopsy were employed in a new integrative active surveillance (AS) paradigm, exemplified by a patient who initially enrolled in AS for 5 years, but later underwent radical prostatectomy upon progression. Metabolic biomarker kPL images detected early progression preceding radiologic and clinical parameters. Final whole-gland histopathology also linked kPL and PI-RADS targets to multifocal tumors, where kPL spatially reflected intratumoral heterogeneity, and high kPL regions correlated with adverse pathologic features such as G4 cribriform pattern, extracapsular extension, and lymphovascular invasion. Results indicated HP 13C may help optimize AS by improving clinical risk calculation.
Purpose
Active surveillance (AS) has become the mainstay for low-risk prostate cancer (PCa) as it safely minimizes treatment-related morbidities1,2. One lingering challenge is to tailor the schedule and intensity of surveillance, which are often both practice-dependent and related to each patient’s risk factors3. Hyperpolarized (HP) 13C-pyruvate MRI can non-invasively differentiate the elevated metabolic biomarker of pyruvate-to-lactate conversion (kPL) in clinically significant PCa versus low-grade, indolent disease4,5, and may therefore lend itself to guide clinical management of AS patients. This case report illustrates a novel strategy for integrating HP 13C MRI into the clinical surveillance and risk stratification paradigm for patients on AS for prostate cancer. The integrated workflow includes serial HP 13C-MRI, kPL-guided fusion biopsy, and finally correlation to the whole-mount surgical pathology.Methods
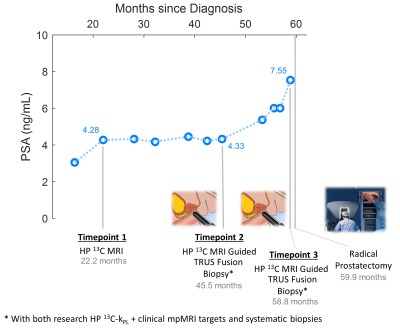
Patient Demographics: The participant was first diagnosed in his late 60s with localized Gleason 3+4, stage cT2a, intermediate-risk6 PCa, with PSA ranging from 2.8 to 4.2. He elected to pursue AS for 5 years, but eventually underwent radical prostatectomy due to clinical evidence consistent with disease progression.Hyperpolarized-13C Pyruvate + mpMRI Exam: Three HP 13C plus standard-of-care (SOC) multiparametric MRI (mpMRI) integrated exams were performed on a clinical 3T magnet (MR750, GE Healthcare, Chicago IL) with 1H-13C dual-tuned endorectal coil, the timeline of which is illustrated in Figure 1. The 13C portion used either a 3D EPSI or EPI sequence with 0.5cc volumetric and 2s temporal resolutions7. The SOC mpMRI followed our institutional protocol (i.e.T2-weighted, DWI, DCE), and was reported using PI-RADS v2.0/2.18. The latter two exams were followed by paired 13C-kPL research targeted biopsies.
For HP imaging, GMP [1-13C]pyruvic acid was polarized in a 5T polarizer (SpinLab, GE Healthcare) for 2-3 hours, yielding 257±13 mM pyruvate solution with 41.0±1.5% polarization, 7.6±0.3 pH, radical concentration 1.1±0.7mM, and temperature 32±1 °C. Injection followed pharmaceutical release in compliance with FDA and IRB approvals9.
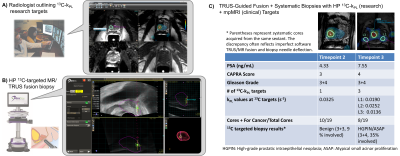
kPL-Guided Research Biopsy and Whole-Mount Histopathology: HP 13C-kPL research biopsies followed previously described methods10. Briefly, kPL maps were generated and uploaded to PACS. Abdominal radiologists with 10+ years of experience outlined suspected kPL targets using pesudocolor fusion overlays (Figure 3A) on a commercial targeting platform (Dynacad, Philips Invivo, Gainsville FL). Using a MR/TRUS fusion biopsy system (Uronav, Philips Invivo), a urologist performed research 13C-targeted biopsy in combination with SOC PI-RADS targets and 12-14 core systematic biopsies (Figure 3B).
Post-prostatectomy, frozen sections and whole-mount histopathology were prepared for the prostate gland, seminal vesicles and pelvic lymph nodes, per institutional practice11. H&E slides were interpreted by a senior genitourinary pathologist with 20+ years of experience.
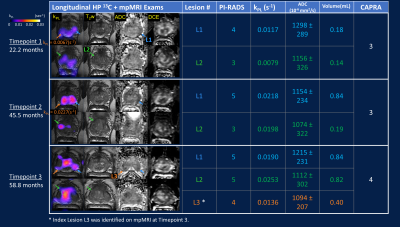
Genomic Test: Frozen sections of index lesion L2 (Figures 2 & 4) were submitted for a commercial PCa genomic risk testing12 (Decipher, Veracyte, South San Francisco, CA).
Results and Discussions
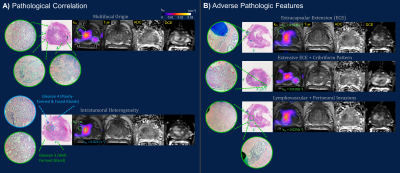
The timeline of HP 13C/SOC mpMRI, paired guided biopsies and radical prostatectomy is illustrated in Figure 1. Intensification of surveillance regimen and eventual definitive treatment were consistent with increased clinical suspicion and evidence of disease progression based on imaging, biopsy and PSA. Both kPL and mpMRI identified multifocal tumors, with serial kPL increase consistent with PI-RADS upgrading on clinical mpMRI (Figure 2). Notably, kPL doubled (L1:0.0117→0.0218s-1, L2:0.0079→0.0198 s-1) during the 22-month interval between timepoints 1 and 2, a year preceding definitive PSA and radiological progression (Timepoint 3, 58.8mo). Additionally, focal mildly elevated kPL (0.0067s-1, orange arrow) could be observed right-mid posterolaterally in retrospect at timepoint 1 without clinical mpMRI correlate at the time. It was not diagnosed clinically (Lesion L3, PI-RADS 4) until timepoint 3 three years later. Indeed, whole-mount surgical pathology found a small Gleason 3+4 focus there (Figure 4A). This suggested that kPL’s early sensitivity to aggressive pathology may complement mpMRI to guide active surveillance.Although targeted 13C-kPL research biopsies found ASAP/HGPIN and benign tissues (Figure 3C), positive tumor cores were present in the same sextant from systematic biopsies. Surgical pathology also confirmed these targets concurred with the three index tumor foci (Figure 4A).
Whole-mount pathology found pT3aN0, multifocal Gleason 3+4 disease, without evidence of lymph node involvement or seminal vesicle invasion. High kPL (>0.014s-1) regions correlated with adverse pathologic features including macroscopic and microscopic extracapsular extension (ECE), G4 cribriform pattern, and lymphovascular invasion, known predictors of poor outcomes (Figure 4B)13,14. As lesion L2 substantially grew in size between timepoints 2 and 3, both kPL,max (Figure 2) and heterogeneity (Figure 4A) increased. Remarkably, high kPL=0.024(s-1) regions medially correlated with predominantly G4 disease, whereas low/undetectable kPL regions laterally correlated with mostly G3 pattern (Figure 4A). This may suggest progression was driven by clonal expansion of aggressive G4 disease15, and reflected the generally higher heterogeneity in larger tumors.
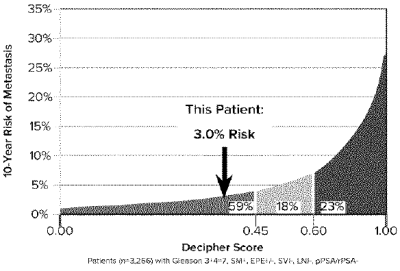
Genomic analysis (Decipher) indicated genomic low-risk with 2.5% likelihood of PCa-specific mortality, putting the patient at 38th percentile among those with similar clinical and pathologic features (Figure 5). This highlights the multivariate nature of PCa risk, and the value of novel metabolic biomarker kPL to improve risk calculations and tailor surveillance intensity.
Conclusions
These results underscore the potential value of HP 13C MRI when integrated with SOC 1H MRI in an active surveillance program.Acknowledgements
This work was supported by grants from the NIH (U01CA232320, U01EB026412, and P41EB013598). We would like to thank Heather Daniel, Francesca De Las Alas, Evelyn Escobar, Mary Frost, Jasmine Hu, Dr. Yaewon Kim, Dr. Philip Lee, Kimberly Okamoto, and Dr. Andrew Riselli for their help with the patient studies, and Ms. Romelyn Delos Santos for her assistance obtaining pathologic samples.References
[1] Cooperberg MR et al., Active surveillance for prostate cancer: progress and promise. J Clin oncol 2015; 29(27), 3669-3676.
[2] Klotz L et al., Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin oncol 2005; 33(3), 272-277.
[3] Cooperberg MR et al., Tailoring intensity of active surveillance for low-risk prostate cancer based on individualized prediction of risk stability. JAMA Oncol 2020; 6(10), 10.1001/jamaoncol.2020.3187.
[4] Nelson SJ et al., Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med 2013; 5(198), 198ra108.
[5] Granlund KL et al., Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab 2020; 31(1), 105-114.
[6] Cooperberg MR et al., The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol 2005; 173(6), 1938-42.
[7] Gordon JW et al., Translation of Carbon‐13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med 2019; 81(4), 2702-2709.
[8] Turkbey B et al., Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019; 76(3), 340-351.
[9] Park I et al., Development of methods and feasibility of using hyperpolarized carbon‐13 imaging data for evaluating brain metabolism in patient studies; Magn Reson Med 2018; 80(3), 864-873
[10] Chen HY et al., Improving Multiparametric MR - TRUS Guided Fusion Prostate Biopsies with Hyperpolarized 13C Pyruvate Metabolic Imaging: Technical Development. Proceedings of the International Society for Magnetic Resonance in Medicine; 30th annual meeting 2020.
[11] Van Leenders G et al., The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. The American journal of surgical pathology 2019; 44(8), e87
[12] Park I et al., Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy; PLoS One 2013; 8:e66855
[13] Magi-Galluzzi C et al., International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease; Modern Pathology 2011; 24(1), 26-38
[14] Kweldam CF et al., Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer; Modern Pathology 2015; 28(3), 457-464
[15] Greaves M et al., Clonal evolution in cancer; Nature 2012; 481(7381), 306-313
Figures