0913
Towards reproducible Arterial Spin Labelling in the myocardium: Impact of blood T1 time and imaging readout parameters1Computer Assisted Clinical Medicine, University Heidelberg, Mannheim, Germany, 2Delft University of Technology, Delft, Netherlands, 3University Aix-Marseille, Marseille, France, 4Barts Heart Centre, London, United Kingdom
Synopsis
Despite promising results, clinical translation of myocardial arterial spin labelling (myoASL) is hampered by insufficient reproducibility and robustness. We investigated the influence of physiological and sequence parameters on FAIR-myoASL in simulations as well as phantom experiments, and developed a correction method based on separately acquired T1 maps. Our simulation and phantom results show acquisition related MBF differences, potentially undermining the reproducibility of myoASL measurements. Inaccuracies between true and reconstruction blood T1, further render the sequence susceptible to heart rate variations, particularly for larger T1 mismatch. Using accurate blood T1 times in reconstruction may improve robustness and reproducibility.
Introduction
The clinical gold standard for detection of myocardial ischemia is first pass myocardial perfusion imaging, where the distribution of an exogenous contrast agent is tracked using T1 weighted imaging1. However, the use of contrast agents limits repeated use and its clinical applicability. Arterial spin labelling (ASL), on the other hand, relies on magnetically labelled blood as an endogenous contrast and has proven effective in quantifying neurovascular perfusion2. In cardiac applications, however, several challenges are faced, such as a complex anatomy and high levels of physiological noise3. Although myoASL and positron emission tomography based myocardial blood flow (MBF) were shown to agree4, insufficient robustness and reproducibility hinder widespread clinical translation. In this work, we sought to investigate the influence of physiological and sequence parameters on the reproducibility of myoASL based perfusion. Further, we sought to develop a correction method based on precedingly acquired T1 maps to increase robustness of myoASL.Numerical Simulations
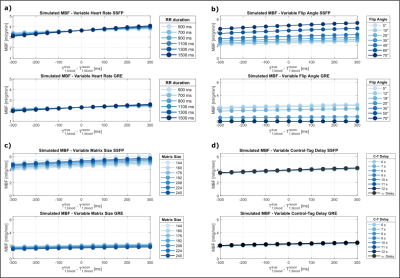
Bloch simulations were performed to investigate the effect of physiological and sequence parameters for bSSFP and spGRE based myoASL. For both imaging readouts, the acquisition flip angle (FA), matrix size, and delay between tag and control image (C-T delay) were varied. Moreover, varying RR intervals, i.e. different effective inversion times (TI), were simulated, with a blood volume fraction of 0.14 and in-flow rate of 0.8ml/g/min.FAIR-myoASL Sequence
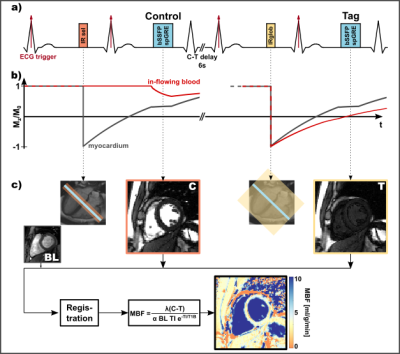
Imaging was performed across two 3T scanners (Magnetom Skyra/Prisma, Siemens Healthineers, Erlangen Germany). For all measurements, a double ECG-gated Flow Alternating Inversion Recovery (FAIR) ASL sequence4,5 was implemented (Fig.1). For the control and tag image, a selective and global adiabatic inversion pulse was applied, respectively, during mid-diastole. Image acquisition (bSSFP/spGRE) followed in mid-diastole of the successive heartbeat.Phantom and in vivo Measurements
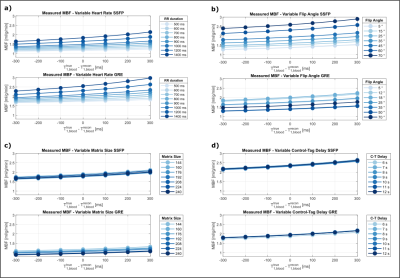
In total, one pair of baseline images (no inversion) and 5/6 (phantom/in vivo) control-tag pairs were acquired with 8mm slice-thickness and 2x2mm/1.7x1.7mm base resolution (phantom/in vivo). MOLLI6 was used for T1 mapping in phantom and in vivo. Phantom experiments were performed with varying RR intervals, FAs and C-T delays in a NiCl2-doped agarose phantom. In-vivo images of 4 healthy subjects (4 male, 39±4.7 years) were obtained with a 6s C-T delay in 12-18s long breath-holds per image pair depending on the heart rate.Data Analysis
After groupwise registration7, mistriggered control-tag image pairs were excluded. Buxton’s model8 was used for MBF reconstruction, where control and tag signal are corrected with respective TI and blood T1 time (T1B). Previous in vivo studies relied on a fixed literature based value for T1B (~1700ms)4,5,9. Here, individual MOLLI-T1B times were used in a second reconstruction method to obtain perfusion maps and septal MBF. In simulations true T1B was varied, while in phantom the model T1 was varied. Mean MBF values were obtained by averaging MBF over all ASL pairs and subjects.Results
Simulated MBF values were constant (3.5/2.3 ml/g/min bSSFP/GRE) for varying heart rates when simulation and reconstruction T1B were identical. When “true” and reconstruction T1B differ, however, the obtained MBF values were highly heart rate dependent. This effect was more pronounced for lower heart rates and is observed in both bSSFP and GRE (Fig. 2a). For higher FAs and matrix sizes, bSSFP based MBF continuously increased. In GRE, MBF decreased to zero for FAs of 30° and beyond (Fig. 2b). For larger matrix sizes, MBF increased in bSSFP and continuously decreased in GRE (Fig. 2c). With varying C-T delay (Fig. 2d), MBF values showed almost no deviation from values simulated with the global inversion pulse acting on fully recovered magnetization (infinite delay). Phantom data depicts similar trends, where MBF difference due to T1B mismatch was stronger for lower heart rates (Fig. 3a). MBF values in bSSFP increased with increasing FA, while in GRE the values increased until 18°, then decreased and stayed constant after 35°. bSSFP based MBF remained constant over the entire range of matrix sizes, whereas GRE based MBF continuously decreased with increasing matrix size. For varying C-T delay, MBF remained largely constant in bSSFP and GRE. In vivo perfusion maps are shown in Fig. 4a for bSSFP and GRE acquisition. Septal MBF was 1.53±1.04ml/min/g(bSSFP) /1.9±1.55ml/min/g(GRE) with fixed T1B, whereas with adaptive T1B MBF was 1.46±0.97ml/min/g (bSSFP) /1.81±1.44ml/min/g (GRE).Discussion
Our simulation and phantom results show that sequence parameters such as FA and matrix size lead to MBF differences, potentially eroding the reproducibility of myoASL. Due to the impact of the imaging readout, effects were particularly pronounced for a large mismatch between reconstruction and true T1B. Moreover, MBF values show a heart rate dependence when true and model T1B differ. Without correction, this may lead to measuring spurious MBF changes in stress and rest conditions. The effect of varying C-T delay on MBF values is negligible in comparison, indicating that 6s may suffice despite long T1B. Both in bSSFP and GRE, simulated and phantom MBF values as well as MBF maps match previously reported perfusion values4,5. The use of adaptive instead of fixed T1B yields slightly more robust reconstruction of myoASL based perfusion.Conclusions
MyoASL shows residual sensitivity to sequence parameters and potentially HR dependence. This may reduce the reproducibility in clinical use and corrupt stress/rest performance. Simulations show that the use of accurate blood T1 time may mitigate this effect for improved robustness.Acknowledgements
No acknowledgement found.References
- Qayyum, A. A., & Kastrup, J. (2015). Measuring myocardial perfusion: the role of PET, MRI and CT. Clinical radiology, 70(6), 576-584.
- Chen, Y., Wang, D. J., & Detre, J. A. (2011). Test–retest reliability of arterial spin labeling with common labeling strategies. Journal of Magnetic Resonance Imaging, 33(4), 940-949.
- Jao, T., & Nayak, K. (2019). Analysis of physiological noise in quantitative cardiac magnetic resonance. Plos one, 14(8), e0214566
- Zun, Z., Wong, E. C., & Nayak, K. S. (2009). Assessment of myocardial blood flow (MBF) in humans using arterial spin labeling (ASL): feasibility and noise analysis. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 62(4), 975-983.
- Do, H. P., Jao, T. R., & Nayak, K. S. (2014). Myocardial arterial spin labeling perfusion imaging with improved sensitivity. Journal of Cardiovascular Magnetic Resonance, 16(1), 1-6.
- Messroghli, D. R., Radjenovic, A., Kozerke, S., Higgins, D. M., Sivananthan, M. U., & Ridgway, J. P. (2004). Modified Look‐Locker inversion recovery (MOLLI) for high‐resolution T1 mapping of the heart. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 52(1), 141-146.
- Tao, Q., van der Tol, P., Berendsen, F. F., Paiman, E. H., Lamb, H. J., & Van Der Geest, R. J. (2018). Robust motion correction for myocardial T1 and extracellular volume mapping by principle component analysis‐based groupwise image registration. Journal of Magnetic Resonance Imaging, 47(5), 1397-1405.
- Buxton, R. B., Frank, L. R., Wong, E. C., Siewert, B., Warach, S., & Edelman, R. R. (1998). A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic resonance in medicine, 40(3), 383-396.
- Lu, H., Clingman, C., Golay, X., & Van Zijl, P. C. (2004). Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magnetic Resonance in Medicine: an Official Journal of the International Society for Magnetic Resonance in Medicine, 52(3), 679-682.
Figures