0908
Open Science Initiative for Perfusion Imaging (OSIPI): Arterial Spin Labeling Imaging and Analysis Lexicon and Reporting Recommendations1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Ghent Institute for Functional and Metabolic Imaging, Ghent University, Ghent, Belgium, 3State University of New York at Binghamton, Binghamton, NY, United States, 4University of Pennsylvania, Philadelphia, PA, United States, 5Department of Radiology, Clínica Universidad de Navarra, Pamplona, Spain, 6University Hospital Hamburg-Eppendorf, Hamburg, Germany, 7Department of Radiology and nuclear medicine, Amsterdam neuroscience, Amsterdam University Medical Center, Amsterdam, Netherlands, 8Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 9University of Southern California, Los Angeles, CA, United States, 10Department of Radiology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States, 11Dept of Brain Repair and Rehabilitation, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom
Synopsis
As part of the Open Science Initiative for Perfusion Imaging (OSIPI), the aim of this work is to develop a Lexicon and Reporting Recommendations for arterial spin labeling (ASL) perfusion MR imaging and analysis. The lexicon describes standardized nomenclature and terminology for ASL acquisition techniques, parameters and physiological constants that are required for quantitative analysis. Additionally, a community-endorsed recommendation for reporting acquisition parameters in publications is provided. Overall, this project aims to improve the clarity and consistency of ASL terminology, which in turn will facilitate comparisons between different studies.
Introduction
The 2015 consensus statement1 published by the ISMRM Perfusion Study Group and the COST Action BM1103 ‘ASL in Dementia’ (www.cost.eu/actions/BM1103/) aimed to encourage the implementation of robust Arterial Spin Labeling (ASL) perfusion MRI for clinical applications and promote consistency across scanner types, sites, and studies. Subsequently, the recommended 3D pseudo-continuous ASL (PCASL) sequence has been implemented by most major MRI manufacturers. However, ASL remains a rapidly and widely developing field, both in terms of improving the accuracy of cerebral blood flow (CBF) quantification and providing other output derivatives in addition to CBF. These advances have greatly expanded the scope of ASL, but also bring further divergence of the technique, particularly in the terminology used, which can lead to confusion and hamper research reproducibility. As part of the Open Science Initiative for Perfusion Imaging (OSIPI), the ASL Lexicon Task Force (osipi.org/task-force-4-1/) has been working on the development of an ‘ASL Perfusion Imaging and Analysis Lexicon and Reporting Recommendations’, aiming: 1) to develop standardized nomenclature and terminology for the broad range of ASL imaging techniques and parameters, as well as the physiological constants required for quantitative analysis, and 2) to provide a community-endorsed recommendation on a minimal list of parameters that should be reported in publications.Methods
The ASL Lexicon task force consists of 11 Perfusion Study Group members with diverse expertise in ASL imaging. The developmental process of the lexicon was:Stage 1 (June 2020 - April 2021): Previously published ASL papers were reviewed by the task force members and comprehensive lists of ASL techniques, acquisition parameters, output derivatives, and physiological parameters required for quantification were compiled. Terminology was harmonized with other community efforts, such as the Brain Image Data Structure (BIDS) extension for ASL2. The Reporting Recommendations were also drafted based on the ASL-BIDS extension, consisting of two recommendation levels: Required: parameters that are essential for meaningful interpretation of the ASL data and for quantitative analysis.Recommended: parameters that could explain specific characteristics or systematic differences between data sets from different ASL sequences.
Stage 2 (June — July 2021): an independent expert panel, composed of members of the ISMRM Perfusion Study Group, provided feedback and comments on the Stage 1 draft. These experts are all currently involved with the development of a new set of ASL consensus review articles (covering multi-delay ASL, velocity-selective ASL, advanced ASL techniques, clinical application and body ASL), and an updated Stage 2 draft was generated based on their feedback.
Stage 3 (June — September 2021): A survey was carried out with the major MRI manufacturers (Canon, Fujifilm, GE, Philips, and Siemens) to identify any potential conflicts or incompatible terminologies and definitions with their current ASL product implementation (PCASL sequence by Canon is work-in-progress as of November 2021).
Stage 4 (November 2021): The draft document was shared with all members of the ISMRM Perfusion Study Group for general feedback and comments. Also, a survey was enclosed so that they could indicate if they agreed with the drafted recommendation categories of the ASL acquisition parameters.
Results
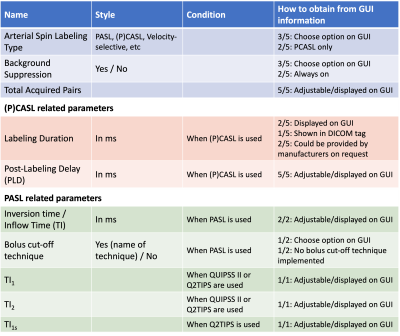
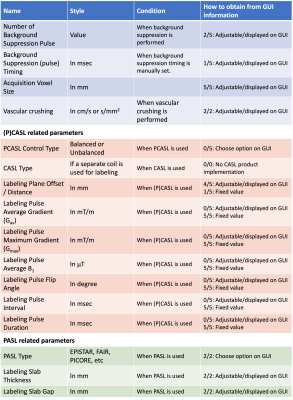
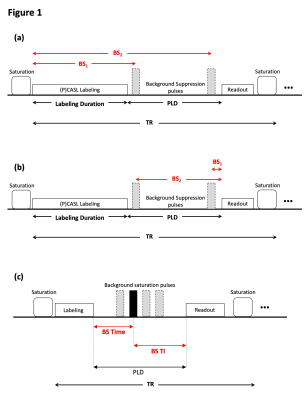
The developed draft document is composed of two main parts: (i) ASL Lexicon and (ii) Reporting Recommendations. The ASL Lexicon consists of seven sections with several subsections (Table 1) in which name, notation, unit, and references are provided. Based on the feedback from the expert panel, the use of terminologies and their definition was harmonized between the ASL Lexicon and the consensus review papers.Tables 2 and 3 show the acquisition parameters that are categorized as “Required” and “Recommended”, respectively, and whether those parameter values can be obtained from the information displayed on the MRI scanner graphical user interface (GUI). All parameters in the “Required” category were found to be displayed on GUI or available on request from the manufacturers. In the “Recommended” category, however, the values of several parameters are not available for some manufacturers. In general, parameter definitions matched well across manufacturers; the main exception related to background suppression timing parameters, which have three different definitions among five manufacturers (see Figure 1).Discussion
As of November 2021, the survey for reporting recommendations is still open for input. After considering the results of the surveys of the manufacturers and Study Group members, we will finalise the list of parameters required and recommended for a publication to be ‘OSIPI compliant’. We encourage authors to include as many of these parameters as possible in ASL study reports.A potential application of the Lexicon and Reporting Recommendations will be the amendment of the DICOM standard for ASL imaging so that parameters not displayed on the GUI can be stored in the DICOM header. Importantly, the content of the lexicon is not intended to be a complete list of all existing techniques, but rather a growing online inventory that will be extended by the community as further methods and improvements are developed and established.In conclusion, we envisage that the developed guidelines will standardize the nomenclature and reporting of ASL perfusion imaging studies and improve reproducibility, reusability, and interoperability of ASL perfusion imaging data.Acknowledgements
The authors would like to thank the expert panel members: Michael Chappell, Luis Hernandez-Garcia, Jia Guo , Thomas Okell, Qin Qin, Eric Wong, Joseph Woods, and MRI manufacturers: Canon Medical Systems Corporation (Valentin Prevost), FUJIFILM Healthcare Corporation (Takashi Tsuneki), GE Healthcare (Suchandrima Banerjee, David Shin), Philips Healthcare (Kim van de Ven), Siemens Healthcare (Josef Pfeuffer, Marta Vidoretta) for providing us with information on their ASL implementation.
YS is supported by the Royal Academy of Engineering under the Research Fellowship scheme (RF/201920/19/236) and core funding from the Wellcome Trust (203139/Z/16/Z). DLT is supported by the UCLH NIHR Biomedical Research Centre and the Wellcome Trust (Centre award 539208). SD is supported by NIH Grants R03 AG063213, R01 NS111115. TL is supported by DFG Grant LI 3030/2-1. MFS is supported by Spanish Ministry of Science and Innovation Universities (grant PI18/00084). TL is supported by the German Research Foundation (DFG), grant number LI-3030/2-1.
References
1. David C. Alsop et al., ‘Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia’, Magnetic Resonance in Medicine 73, no. 1 (January 2015): 102–16, https://doi.org/10.1002/mrm.25197.
2. Clement, Patricia, Marco Castellaro, Thomas W. Okell, David L. Thomas, Pieter Vandemaele, Sara Elgayar, Aaron Oliver-Taylor, et al. 2021. “ASL-BIDS, the Brain Imaging Data Structure Extension for Arterial Spin Labeling.” PsyArXiv. October 25. doi:10.31234/osf.io/e87y3.
Figures