0907
Combined optimisation of delays and echo times for blood-brain barrier permeability measurements using multi-TE pCASL MRI1Department of Engineering Science, Institute of Biomedical Engineering, Nottingham, United Kingdom, 2Nuffield Department of Clinical Neurosciences, University of Oxford, Wellcome Centre for Integrative Neuroimaging, FMRIB, Oxford, United Kingdom, 3School of Medicine, University of Nottingham, Radiological Sciences, Division of Clinical Neurosciences, Nottingham, United Kingdom, 4School of Medicine, University of Nottingham, Sir Peter Mansfield Imaging Centre and Mental Health & Clinical Neurosciences, Nottingham, United Kingdom
Synopsis
Blood-brain barrier permeability to water has been measured as the exchange time (Texch) between intra- and extra-vascular spaces using multi-TE arterial spin labelling (ASL). This relies on small signal changes associated with labelled blood water delivery, making it challenging to achieve high accuracy in short yet clinically-desirable scan durations. In this study, we used an optimal sampling framework to generate pseudo-continuous ASL protocols that were jointly optimised for delays and echo times. Simulation showed that while optimised protocols had overall improvements in parameter estimation compared to conventionally even-sampled protocols, accurately estimating Texch could not be achieved without sacrificing CBF accuracy.
Introduction
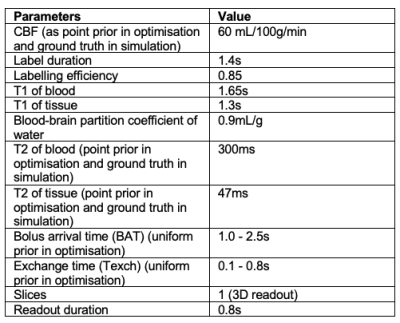
Disruption of the blood-brain barrier (BBB) is thought to be a precursor to vascular changes in many cerebral pathologies, such as stroke1, multiple sclerosis2, and neurodegenerative diseases3. The exchange time ($$$T_{exch}$$$) for water molecules to transport from the intravascular space to extravascular space has been measured as an indicator of BBB permeability using the T2-relaxation-under-spin-tagging (TRUST) pseudo-continuous arterial spin labelling (pCASL) MRI4-5, where a T2-preparation module was inserted before image readout to modify the effective TE (eTE). However, such measurements have been challenging due to the intrinsically low SNR of the ASL imaging technique and the need for a short scan duration for clinical application (e.g., five minutes). Hence, we exploited an optimal sampling framework that aimed for combined optimisation of post-labelling delays (PLD) and TE, so as to achieve better estimations of $$$T_{exch}$$$ while preserving the accuracy of CBF estimation.Methods
OptimisationCombined optimisation of PLD and eTE was achieved using a general optimisation framework for ASL experiments6, which is based on maximising the Fisher Information Matrix (FIM) to minimise the parameter variance specified by Cramer-Rao Lower Bound. The parameters of interest in optimisation were $$$\theta = {CBF, BAT, T_{exch}, T2b, T2t}$$$. Other parameters used in the optimisation are listed in Table 1. A two-compartment kinetic model separating the intra- and extra-vascular signal contributions was used to derive the sensitivity functions of each parameter7. The L-optimality criterion was considered so that only a subset of parameters was optimised for, while treating other parameters as confounds6. The resulting optimised protocols were $$$CBFT_{exch}opt$$$ and $$$CBFopt$$$, where the former was optimised for both CBF and $$$T_{exch}$$$ and the latter serves as a reference that only optimised for CBF.
Validation
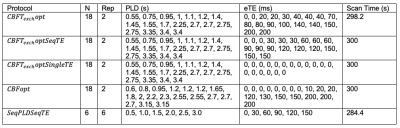
To validate the performance of parameter estimation by optimised protocols, pCASL signals using the two-compartment model were simulated and added Gaussian white noise to achieve an SNR of 2, imitating a low-SNR scenario. Five protocols were included in the validation study, including the newly-acquired optimised protocols, $$$CBFT_{exch}opt$$$ and $$$CBFopt$$$. $$$CBFT_{exch}optSeqTE$$$ shares the same PLD timings as $$$CBFT_{exch}opt$$$ but with a sequential eTE pattern commonly used in literature. $$$CBFT_{exch}optSingleTE$$$ also has the same PLD timings as $$$CBFT_{exch}opt$$$ but with zero eTEs, corresponding to a conventional optimised pCASL protocol8. $$$SeqPLDSeqTE$$$ is an evenly-spaced PLD/eTE sequence, corresponding to a conventional multi-delay multi-echo protocol. Their PLD and eTE timings are listed in Table 2. The bolus arrival time (BAT) varied between 1.0s to 2.5s, while $$$T_{exch}$$$ varied between 0.1s to 0.8s. Other simulation parameters can be found in Table 1. The signals were then fitted with the same model using the $$$nlinfit$$$ function in MATLAB (The MathWorks, Natick, MA). Relative estimation errors of CBF, BAT, and $$$T_{exch}$$$ were obtained and averaged over 10000 simulations of uniformly sampled parameter space. Root mean-square error (RMSE) maps over 1000 simulations across the simulated BAT and $$$T_{exch}$$$ ranges at 0.05s intervals were generated for each protocol.
Results and Discussion
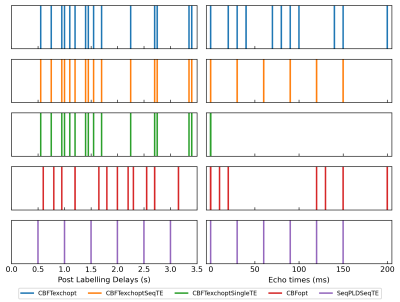
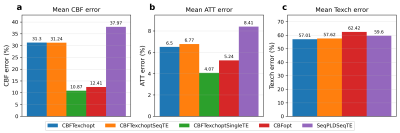
A schematic of PLD and eTE timings of optimised protocols is shown in Figure 1. Protocols that are optimised for CBF measurements ($$$CBFT_{exch}opt$$$ and $$$CBFopt$$$) have more long-PLDs than the $$$SeqPLDSeqTE$$$ protocol, since the SNR is highest around the peak of the kinetic curve for robust CBF estimation. $$$CBFT_{exch}opt$$$ has more long-eTEs than $$$CBFopt$$$ for separating the two compartments and hence targeted at $$$T_{exch}$$$ estimation.Figure 2 shows the relative estimation errors of CBF, BAT, and $$$T_{exch}$$$ by the five protocols. For mean $$$T_{exch}$$$ error, $$$CBFT_{exch}opt$$$ was lower than $$$CBFopt$$$ by ~5.4%, and marginally lower than the conventional sequential TE protocols $$$CBFT_{exch}optSeqTE$$$ and $$$SeqPLDSeqTE$$$. $$$CBFT_{exch}optSingleTE$$$ was not able to estimate $$$T_{exch}$$$ accurately for failing to separate compartmental signals using single TE. For CBF estimation, $$$CBFopt$$$ had the least mean error among multi-TE protocols, ~19% lower than $$$CBFT_{exch}opt$$$. However, it had a higher mean error than the single-TE sequence, presumably due to higher SNR when eTE equals zero.
RMSE maps of CBF, BAT and $$$T_{exch}$$$ in the simulated BAT and $$$T_{exch}$$$ ranges are shown in Figure 3. The RMSEs of all three parameters for all protocols were quite consistent across the simulated $$$T_{exch}$$$ range, while $$$CBFT_{exch}opt$$$, $$$CBFT_{exch}optSeqTE$$$ and $$$SeqPLDSeqTE$$$ had considerably higher RMSE when BAT > 2.3s, suggesting the reduced performance by the OSS around parameter boundaries.
The results indicated that optimised protocols had overall improved estimation performance compared to conventional even-sampling protocols. However, the trade-off for reducing $$$T_{exch}$$$ estimation error by ~5.4% was a nearly four-fold increase of CBF estimation error, demonstrating the limited ability to obtain sufficient estimation accuracy in both CBF and $$$T_{exch}$$$. This may be alleviated using time-encoded ASL or multi-echo readout schemes to fit more repeats within the scheduled time, or extending the scan duration to achieve higher SNR. Also, our current observations were based on simulation, future work should include in vivo experiments to further validate the performance of optimised protocols in reality.
Conclusion
We applied an optimal sampling framework in TRUST pCASL to obtain protocols that were jointly optimised for PLD and eTE. The optimised protocols provided overall improvements in parameter estimation than conventional evenly-spaced protocols. However, accurately estimating $$$T_{exch}$$$ could not be achieved without sacrificing CBF accuracy.Acknowledgements
This work received funding from the Engineering and Physical Sciences Research Council (EP/P012361/1).References
1. Kassner A, Merali Z. Assessment of blood–brain barrier disruption in stroke. Stroke. 2015 Nov;46(11):3310-5.
2. Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Multiple Sclerosis Journal. 2003 Dec;9(6):540-9.
3. Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood–brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. Cell transplantation. 2007 Mar;16(3):285-99.
4. Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2‐relaxation‐under‐spin‐tagging MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Aug;60(2):357-63.
5. Schmid S, Teeuwisse WM, Lu H, van Osch MJ. Time-efficient determination of spin compartments by time-encoded pCASL T2-relaxation-under-spin-tagging and its application in hemodynamic characterization of the cerebral border zones. Neuroimage. 2015 Dec 1;123:72-9.
6. Woods JG, Chappell MA, Okell TW. A general framework for optimizing arterial spin labeling MRI experiments. Magnetic resonance in medicine. 2019 Apr;81(4):2474-88.
7. Gregori J, Schuff N, Kern R, Günther M. T2‐based arterial spin labeling measurements of blood to tissue water transfer in human brain. Journal of magnetic resonance imaging. 2013 Feb;37(2):332-42.
8. Zhang LX, Woods JG, Okell TW, Chappell MA. Examination of optimized protocols for pCASL: Sensitivity to macrovascular contamination, flow dispersion, and prolonged arterial transit time. Magnetic Resonance in Medicine. 2021 May;86(4)2208-19.
Figures