0903
Optimization and evaluation of inversion pulses for background suppressed pseudo-Continuous Arterial Spin Labeling at 7T1Laboratory of FMRI Technology (LOFT), Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Institute of Medical Engineering, Graz University of Technology, Graz, Austria, 3Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
Synopsis
Background suppression (BS) of pseudo-Continuous Arterial Spin Labeling (pCASL) needs to be optimized at 7T to ameliorate the ASL signal loss caused by the low inversion efficiency (IE) of BS due to B1 inhomogeneity. In this study, the original HS pulse used as BS at 3T was assessed at 7T, and four non-selective inversion pulses, including HS, WURST, OPTIM, and pTx adiabatic, were optimized and evaluated in phantom and in-vivo studies. Less than 80% IE achieved by the original HS pulse was improved to 85% by optimized pulses. Markedly higher tSNR was reported in pCASL with the optimized pulses.
Background
Background suppression (BS) has been recommended for pseudo-Continuous Arterial Spin Labeling (pCASL) at 3T1 with high inversion efficiency (IE) (~95%)2, and improved temporal signal-to-noise ratio (tSNR). At 7T, however, the lower IE of BS pulses due to transmit B1 inhomogeneity may lead to loss of ASL signal. While recent studies optimized labeling parameters of pCASL at 7T3, the BS pulse itself was not optimized, and IE remains unknown. In this study, we optimized several non-selective inversion pulses for 7T including Hyperbolic Secant (HS)4, wideband uniform rate smooth truncation (WURST)5, OPTIM6, and adiabatic pulse with parallel transmission (pTx), which were compared to the original HS pulse used at 3T7.Methods
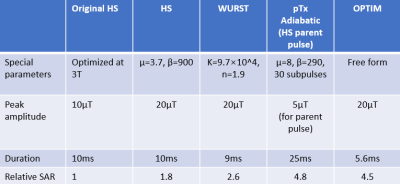
Based on the definitions4,5, A wide range of μ (2-9) and β (400-1800) for HS and k (50-150×104) and n (0.1-10) for WURST were assessed. Besides, the pulse duration (5-25ms) and the peak amplitude (5-20μT) were optimized. B1 scaling factors (0.3-1.1) and B0 offsets (+/-280Hz) were determined according to the B1 and B0 maps acquired from healthy subjects at 7T. The optimal parameters were determined by minimizing Root-mean-square error (RMSE) between the simulated inversion profile (T1blood=2087ms and T2blood=68ms) and the ideal inversion across all B1 factors and B0 offsets.The OPTIM inversion pulse was designed as a free form RF pulse using ensemble time optimal control techniques6,7. Assuming an operation peak amplitude of 20μT, we designed for a B1-robustness from 0.3-1.1 of the nominal amplitude and B0-robustness for +/-500Hz. The optimization itself was started from a random RF pulse with an initial duration of 15ms.
A pTx adiabatic pulse was designed following9 and was subject to the hardware limitations. A HS pulse was optimized as the parent pulse, and then was divided into multiple piece-wise constant elements. pTx sub-pulses were designed using spatial domain method10 with SPINS k-space trajectory11 to achieve equivalent Beffective of these elements.
Phantom and in-vivo experiments were performed on Siemens 7T Terra with a Nova 8Tx/32Rx head coil. A Sagittal and an Axial slice at the center position for phantom, and a center Sagittal slice and two Axial slices covering superior and inferior positions, respectively, for in-vivo scans were acquired. The inversion pulses were immediately followed by 2D Turbo-FLASH (TFL) readout to measure IE (FOV=210×192mm, matrix size=96×88, slice thickness=5mm, TR=150ms, TE=1.38ms, FA=8°). Perfusion images were acquired by pCASL (TR=6000ms, labeling duration=1000ms, post labeling delay=1500ms, RF duration=300μs, RF gap=190μs, Gave/Gmax=0.6/6mT/m, and 38 measurements for 3:50mins) with 3D TFL readout (FOV=210×192×75mm, matrix size=96×88×10, slice thickness=5mm, TR=176ms, TE=1.49ms, FA=8°). For pTx pulse, RF duration and RF gap were adjusted to 500μs and 300μs, respectively, due to hardware limitations. Two identical inversion pulses were placed between the labeling and TFL acquisition according to7 for each scan.
IE maps were calculated as the ratio between two images acquired with and without inversion pulses to analyze IE and inversion homogeneity using Coefficient of Variation (CV). CBF was quantified based on12 with assumed IE=0.95. IE of BS in pCASL was estimated as the square root of the ratio of global CBF with BS to that without BS. tSNR was calculated in GM perfusion signals.
Results and discussion
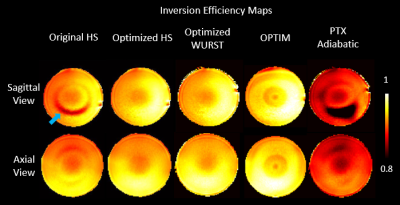
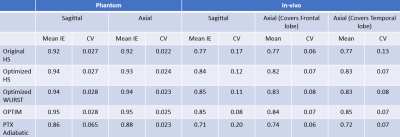
The optimized parameters of inversion pulses are listed in Table 1. Figure 1 and 2 show Sagittal and Axial IE maps of different inversion pulses in the phantom and in-vivo scans, respectively. Low IE, especially in the ring region (blue arrow) in Fig.1 and temporal lobes (white arrows) in Fig.2(a) and (c) which suffered from insufficient B1, was observed for the original HS, and was significantly improved by optimized HS, optimized WURST, and OPTIM. Among the latter three pulses, OPTIM achieved the highest IE in low B1 regions and the central region of the phantom where excessive B1 was delivered, while HS and WURST showed similar improvements. However, despite of the slightly increased IE in temporal lobes demonstrated in Fig. 2(c), pTx adiabatic pulse performed worse than the original HS potentially due the lengthy pulse duration.Table 2 lists mean IE and CV values of all conditions. On average of all slices, IE of 0.92 for the phantom and 0.77 for in-vivo were reported for the original HS. While the optimized pulses except for pTx adiabatic had higher IE and similar or lower CV than the original HS, OPTIM outperformed others with IE of 0.95 in the phantom and 0.85 in in-vivo scans. Up to 50% reduction of CV was reported for the optimized pulses compared to the original HS, demonstrating improved inversion homogeneity.
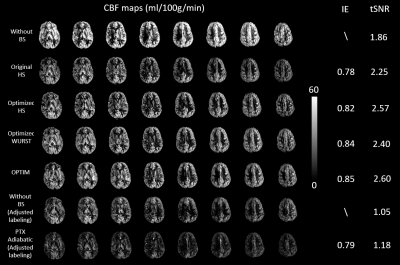
Figure 3 shows CBF maps acquired with different inversion pulses, and lists IE and tSNR. CBF was significantly underestimated because of the imperfect inversion. IE of 0.78 was reported for the original HS. After optimization, IE of HS, WURST, OPTIM, and pTx adiabatic were improved to 0.82, 0.84, 0.85, and 0.79, respectively. With optimized BS pulses, tSNR were markedly higher than that obtained without BS and with the original HS.
Conclusion
We optimized and evaluated several BS pulses for pCASL at 7T. Our preliminary results show that the original HS at 3T achieved an IE less than 80%, which can be improved to 85% after optimization for 7T pCASL. OPTIM showed the best performance among all candidate pulses.Acknowledgements
This work was supported by National Institute of Health (NIH) grant S10-OD025312, R01-EB032169 and R01-EB028297. We thank Armin Rund (Institute for Mathematics and Scientific Computing, Karl-Franzens University Graz, Graz, Austria) for providing the trust-region semi-smooth quasi-Newton optimization framework.References
[1] Alsop, David C., et al. "Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia." Magnetic resonance in medicine 73.1 (2015): 102-116.
[2] Garcia, Dairon M., Guillaume Duhamel, and David C. Alsop. "Efficiency of inversion pulses for background suppressed arterial spin labeling." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 54.2 (2005): 366-372.
[3] Wang, Kai, et al. "Optimization of pseudo‐continuous arterial spin labeling at 7T with parallel transmission B1 shimming." Magnetic Resonance in Medicine (2021).
[4] Silver, Michael S., Richard I. Joseph, and David I. Hoult. "Highly selective π2 and π pulse generation." Journal of Magnetic Resonance (1969) 59.2 (1984): 347-351.
[5] Kupce, E., and R. Freeman. "Adiabatic pulses for wideband inversion and broadband decoupling." Journal of Magnetic Resonance, Series A 115.2 (1995): 273-276.
[6] Graf, Christina, et al. “Time optimal control based design of robust inversion pulses.” In Proceedings of the International Society of Magnetic Resonance in Medicine (ISMRM), 2021.
[7] Rund, Armin, et. Al, “Simultaneous multislice refocusing via time optimal control.” Magnetic Resonance in Medicine 80.4 (2018): 1416-1428.
[8] Shao, Xingfeng, et al. "A constrained slice‐dependent background suppression scheme for simultaneous multislice pseudo‐continuous arterial spin labeling." Magnetic resonance in medicine 79.1 (2018): 394-400.
[9] Jang, Albert, et al. "Designing 3D selective adiabatic radiofrequency pulses with single and parallel transmission." Magnetic resonance in medicine 79.2 (2018): 701-710.
[10] Grissom, William, et al. "Spatial domain method for the design of RF pulses in multicoil parallel excitation." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 56.3 (2006): 620-629.
[11] Malik, Shaihan J., et al. "Tailored excitation in 3D with spiral nonselective (SPINS) RF pulses." Magnetic resonance in medicine 67.5 (2012): 1303-1315.
[12] Wang, Yi, et al. "Simultaneous multi-slice Turbo-FLASH imaging with CAIPIRINHA for whole brain distortion-free pseudo-continuous arterial spin labeling at 3 and 7 T." Neuroimage 113 (2015): 279-288.
Figures