0901
4D flow MRI: Ex-vivo swine model allows distinction between flow patterns induced by aortic valve replacement and surgical pathway alone.1Department of Radiology and Nuclear Medicine, Universität zu Lübeck, Lübeck, Germany, 2Department of Cardiac and Thoracic Vascular Surgery, Universität zu Lübeck, Lübeck, Germany, 3Department of Radiology, University of Wisconsin, Madison, WI, United States
Synopsis
Hemodynamic outcome after aortic valve replacement (AVR) seems dependent on the type of replacement. The impact of the surgical pathway (aortotomy) itself on hemodynamics is still unclear. We proposed an ex-vivo swine model for comprehensive evaluation of aortic hemodynamics after different types of AVR with 4D flow MRI. Postoperative flow changes could be attributed not only to the implanted valve but also to the aortotomy. The new neocuspidization Ozaki procedure compared favorably to a biological valve as it induced fewer secondary flow patterns. Among the three different AVR methods, the mechanical valve allowed for hemodynamics that most closely approached physiology.
Introduction:
Aortic valve replacement (AVR) is one of the most frequently performed cardiac procedures. There exist different options for surgical AVR including mechanical and biological valve prostheses1,2. Moreover, Ozaki aortic valve neocuspidization3 is a new procedure for reconstructing the aortic valve using the patient’s own pericardium. In children, the valve cusps can be implanted oversized to allow growth of the native aortic annulus4. Like biological valves, Ozaki procedure does not necessitate anticoagulation4. For surgical AVR, an incision in the ascending aorta is needed to gain access to the valve. Little is known about the impact of this surgical pathway and of different types of AVR on aortic hemodynamics. However, hemodynamics play a vital role in effective blood propulsion, aortic vessel wall remodelling5, and valve as well as cardiac function6,7. The purpose of this work was to establish an ex-vivo model to comprehensively evaluate the impact of the surgical procedure alone and in combination with different valve replacement strategies on aortic hemodynamics.Methods:
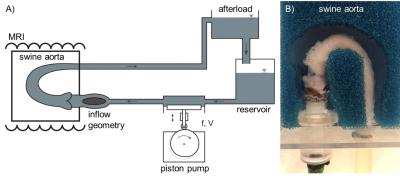
Ex-vivo model: Six fresh swine aortas were dissected from the left ventricular outflow tract (LVOT) to the distal descending thoracic aorta and anastomosed to an in-house developed piston pump. Blood-mimicking fluid was pumped at 3l/min and 60bpm. In all 6 specimens, a cardiac surgeon performed a sham surgery by dissecting the ascending aorta exposing the aortic valve without replacing the valve (ShamSur). He sewed the incision as per clinical standard. In a second surgery, he undid the stitching and performed routine AVR with either a biological valve (BioV, Perimount MagnaEase, 21mm, Edwards Lifesciences, USA; N=2), a mechanical valve (MecV, Standard Masters, 21mm, St. Jude Medical, USA; N=2), or the Ozaki procedure (Ozaki, N=2).MRI acquisition: 4D flow MRI was acquired at 3Tesla (Achieva, Philips, The Netherlands) with the following parameters: TR=4.0ms, TE=2.8ms, resolution=2.45x2.5x2.5mm³, reconstructed to 1.24x1.24x1.45mm³, retrospective ECG gating synchronized with the pump, temporal resolution: 40ms, SENSE acceleration factor=2.2, acquisition time=4min. Each aorta was imaged 3 times: prior to surgery, after sham surgery, and after valve replacement.
Analysis: Reconstruction and postprocessing included correction for background phase offsets, Maxwell terms and Eddy currents. Using GTFlow (GyroTools, Switzerland), peak velocity was quantified, and hemodynamics visualized using pathlines. Secondary flow patterns deviating from main flow were graded on a Likert scale according to their diameter in relation to the vessel cross section as small, medium and large5. Statistical analysis was descriptive.
Results:
4D flow MRI was successfully acquired for all specimens. We could observe physiological systolic flow in all aortas pre surgery without any secondary flow patterns. After sham surgery, one small to medium sized secondary flow pattern developed in the ascending aorta at the site of the incision in all 6 cases, respectively (Tab.1). All types of AVR did cause more or more pronounced secondary flow patterns than sham surgery alone. The highest number of secondary flow patterns was observed distal to the biological valve (N=3, Fig.1), followed by Ozaki (N=2, Fig.2). Like sham surgery, mechanical valves presented with only one secondary flow pattern. However, this was more pronounced (Fig.3). Distal to biological valves, there was a narrow ejection jet towards the outer curvature of the ascending aorta. This was associated with increased peak velocity compared to pre surgery, mechanical valves and Ozaki procedure (PreSur=89.6±59 cm/s, ShamSur=57.6±17.9cm/s, MecV=69.2±27.6cm/s, BioV=110.9±27.1cm/s, Ozaki=69.8±5.3cm/s).Discussion:
We successfully established an ex-vivo swine model for testing aortic valve replacement surgery. We found that the surgical pathway itself altered normal hemodynamics. More pronounced flow alterations were induced by AVR, especially by biological valves and to a lesser extent by the Ozaki procedure. Mechanical valves interfered least with aortic hemodynamics. Currently, little is known on the impact of the Ozaki procedure on aortic hemodynamics. In our study, the Ozaki procedure was not associated with increased peak velocity distal to the valve as opposed to biological valves. Both AVR techniques use pericardium as valve cusps– either the patient’s own (Ozaki)4 or bovine pericardium. The main difference is the frame of biological valves to maintain their shape. This frame narrows the effective opening area. In contrast, in the Ozaki procedure, the pericardium is sewed directly into the native aortic annulus and apparently without narrowing the outflow. Previous studies have shown disturbed flow after valve replacements in CFD simulations8, in silicone models9, and patients after AVR. We could substantiate previous findings that biological valves induce unfavorable hemodynamics compared to mechanical valves. This study adds to the body of knowledge by demonstrating that even the surgical pathway alone alters hemodynamics. A limitation of the model is a set up-related valve insufficiency that did not allow analysis of diastolic flow patterns and valve competency. Moreover, volumetric output of the pump was relatively low to minimize leakage from the vessel. The proposed ex-vivo swine model could help optimizing the surgical pathway as well as valve prostheses to minimize disturbance of hemodynamics. The clinical impact of these findings needs to be determined in long-term follow-up studies in patients.Conclusion:
We have demonstrated that alone the incision in the aorta for aortic valve replacement surgery alters aortic hemodynamics. The new Ozaki procedure induced more secondary flow patterns than mechanical valves but fewer than biological valves.Acknowledgements
No acknowledgement found.References
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al., 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021.
2. Krane M, Wirth F, Boehm J, Lange R, Do we need to rethink treatment of aortic valve pathologies in younger patients? European Journal of Cardio-Thoracic Surgery, Volume 60, Issue 1, July 2021, 46–47.
3. Ozaki S, Kawase I, Yamashita H, Uchida S, Nozawa Y, Matsuyama T, Takatoh M, Hagiwara s, Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease, Interactive CardioVascular and Thoracic Surgery, Volume 12, Issue 4, April 2011, 550–553.
4. Cuttone F, Alacoque X, Leobon B, Karsenty C, Guitarte A, Dulac Y, Chausseray G, Acar P, Hadeed K. Aortic valve reconstruction in children: A new string to our bow. Arch Cardiovasc Dis. 2019 Nov;112(11):653-656.
5. Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995 Jul;75(3):519-60.
6. Bellhouse BJ, Bellhouse FH, Reid KG. Fluid mechanics of the aortic root with application to coronary flow. Nature. 1968;219 (5158):1059‐1061.
7. Oechtering TH, Sieren MM, Hunold P, Hennemuth A, Huellebrand M, Scharfschwerdt M, Richardt D, Sievers HH, Barkhausen J, Frydrychowicz A. Time-resolved 3-dimensional magnetic resonance phase contrast imaging (4D Flow MRI) reveals altered blood flow patterns in the ascending aorta of patients with valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2019;1-13.
8. Hellmeier F, Nordmeyer S, Yevtushenko P, et al. Hemodynamic evaluation of a biological and mechanical aortic valve prosthesis using patient‐specific MRI‐based CFD. Artif Organs. 2018;42(1): 49‐57.
9. Oechtering TH, Sieren M, Schubert K, Schaller T, Scharfschwerdt M, Panagiotopoulos A, Fujita B, Auer C, Barkhausen J, Ensminger S, Sievers HH, Frydrychowicz A. In vitro 4D Flow MRI evaluation of aortic valve replacements reveals disturbed flow distal to biological but not to mechanical valves. J Card Surg. 2019;1–6.
Figures