0900
Automatic analysis of cardiovascular magnetic resonance 2D phase contrast CMR imaging1School of Biomedical Engineering and Imaging Science, King's College London, London, United Kingdom, 2Department of Adult and Paediatric Cardiology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom, 3Department of Cardiology, Heart and Lung Division, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Cardiovascular magnetic resonance (CMR) based flow volume quantification in the great thoracic vessels is used in the assessment of several cardiovascular diseases such as valvular regurgitation, cardiac shunts and vascular health. Clinically, flow volume quantification is often performed based on semi-automatic segmentation of a vessel throughout the cardiac cycle in a manually positioned 2D phase-contrast (PC) CMR plane. In this work, we proposed a quality-controlled AI-based framework for automatic flow quantification from a full CMR scan that includes automated view selection. Results show high accuracy in view selection and excellent agreement between manual and automated flow quantification analysis.
Introduction
The assessment of blood flow parameters is important for the study of cardiovascular function and clinical evaluation of cardiovascular disease. Cardiac Magnetic Resonance imaging (CMR) allows quantification of blood flow, by assessing the phase shift in moving particles. Accurate and reliable quantification is essential for correct diagnosis and could guide treatment decisions. Technical aspects such as field strength, vendor platforms and imaging protocol influence CMR results. CMR flow analysis is currently performed semi-automatically by experienced imaging cardiologists. However, this process is tedious, time-consuming and prone to subjective errors. Recently, artificial intelligence (AI) models have shown remarkable success in automating many medical image analysis tasks1-2.In this work, we proposed an AI-based framework for automated detection of flow images and subsequent analysis of the images to obtain the desired flow parameters.
Methods
The framework we present is composed of four steps: 1) view selection aimed at identifying conventional phase-contrast (PC) views; 2) automated segmentation of PC images; 3) quality control (QC) of the output of the segmentation network; and 4) parameter extraction (see Figure 1 for summary):- View Selection: The first part of our framework aimed to identify the standard 2D PC views used for analysis of cardiac function and hemodynamics from a CMR acquisition. We used the DenseNet3 network, a state-of-the-art classifier, to classify between the ascending aorta, pulmonary trunk and other views. Prior to training, all images were cropped to a standard size of 192x192 pixels, and the network was trained for 200 epochs with cross entropy loss.
- Automated segmentation: We used the ‘nnU-Net’ framework4 to segment the ascending aorta and pulmonary artery in the ascending aorta and pulmonary trunk views respectively.
- Quality control: We examined peak flow values, positive vs negative flow ratio and ejection times against expected values.
- Parameter extraction: We quantify peak flow, total forward, backward and net flow, as well as the onset of ejection (valve opening time), cessation of ejection (valve closure time) and net ejection time. Additionally, we quantify area change (distensibility) during the ejection phase.
Materials and Experiments
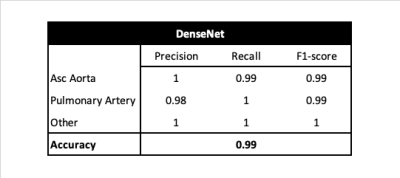
Datasets: This is a retrospective multivendor study conducted on a large CMR dataset from the UK Biobank and Guy’s and St Thomas’ NHS Foundation Trust (GSTFT) London that include the full spectrum of cardiac disease phenotypes. Images were acquired on 1.5T and 3.0T Philips and Siemens CMR scanners using a large variety of protocols, with variable voxel- and image-size, acquisition techniques and under-sampling factors. For view classification, 600 cases were used; and for the automated segmentation, 620 ascending aorta and 340 pulmonary artery cases were used. In both cases data were split 80/10/10% for training/validation/test. All data were manually classified and annotated though the full cardiac cycle by multiple physicians, and an additional review of all segmentations was performed to ensure high quality of the dataset.View Selection: Precision, recall, F1-score and accuracy in the test database of the DenseNet network are shown in Figure 2.
Automated segmentation: The average Dice score in the test database is shown in Figure 3.
Parameter extraction: There was a strong correlation between automatically and manually obtained values in aortic peak flow and net flow (peak flow, r= 0.994; net flow r = 0.996) and in pulmonary peak flow and net flow (peak flow, r= 0.997; net flow r = 0.995). Bland-Altman plots for agreement between the automated and manual analysis are shown in Figure 4. There was no significant bias for aortic or pulmonary peak flow or net flow.
Discussion and Conclusions
Automation of CMR 2D PC flow analysis comes with a number of challenges, including correct view selection, segmentation of the vessels over the cardiac cycle, correct computation of flow volume and peak velocity quantification, and QC of the results.In this work, we proposed a robust end-to-end AI-based pipeline for automatic flow quantification, that offers a solution for each of the challenges encountered. The pipeline has three major steps: view selection that detects the two main sequences used for flow quantification; automatic segmentation of these two sequences; QC to ensure that the output segmentations are correct; and finally, parameter estimation.
We show that our proposed AI-based framework, which combines training on a large-scale multi-domain CMR database with two state-of-the-art AI algorithms, allows us to robustly deal with routine clinical data from multiple centers, vendors, and field strengths and have excellent agreement between manual and automatic analysis. This is a fundamental step for the clinical translation of AI algorithms. Future work aims to integrate this framework with our previous pipeline for automatic analysis of cine CMR sequences1, allowing for fully automatic analysis of cardiac function and hemodynamics, and extending our view-detection algorithm to a larger set of flow planes (such as descending aorta and branch pulmonary arteries) that are less often used.
Acknowledgements
This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering (WT203148/Z/16/Z), and by the NIHR Cardiovascular MedTech Co-operative award to the Guy’s and St Thomas’ NHS Foundation Trust. This research has been conducted using the UK Biobank Resource (application number 17806).References
1. Ruijsink, Bram, et al. "Fully automated, quality-controlled cardiac analysis from CMR: validation and large-scale application to characterize cardiac function." Cardiovascular Imaging 13.3 (2020): 684-695.
2. Bernard, Olivier, et al. "Deep learning techniques for automatic MRI cardiac multi-structures segmentation and diagnosis: Is the problem solved?." IEEE transactions on medical imaging 37.11 (2018): 2514-2525.
3. Huang, Gao, et al. "Densely connected convolutional networks." Proceedings of the IEEE conference on computer vision and pattern recognition. 2017.
4. Isensee, Fabian, et al. "nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation." Nature methods 18.2 (2021): 203-211.
Figures