0895
Analysis of Fluid-Structure Stability from 4D Flow MRI to Predict Aortic Dilation
Tom Y Zhao1, Guy Elisha1, Ethan M I Johnson2, Sourav Halder1, Ben C Smith2, Bradley D Allen2, Michael Markl2, and Neelesh A Patankar1
1Northwestern University, Evanston, IL, United States, 2Northwestern University, Chicago, IL, United States
1Northwestern University, Evanston, IL, United States, 2Northwestern University, Chicago, IL, United States
Synopsis
We present a novel analysis of 4D flow MRI in the thoracic aorta that identifies fluid structure instabilities, which are elevated in a cohort of 117 suspected-aortopathy patients as compared to 100 healthy subjects, and which are predictive of later complications shown in a follow-up analysis for 72 patients in the cohort.
Introduction
Current guidelines for clinical management of patients with aortic dilatation entail regular imaging, with maximal diameter and/or interval diameter changes being key metrics for identifying patients at higher risk for complications, such as dissection 1. However, it remains a challenge to distinguish between patients with stable aortic dilatation and those who may experience further growth and may require surgical intervention. Here, we present a novel analysis of 4D flow MRI in the thoracic aorta that identifies fluid structure instabilities, which are elevated in a cohort of 117 suspected-aortopathy patients as compared to 100 healthy subjects, and which are predictive of later complications shown in a follow-up analysis for 72 patients in the cohort.Methods
An institutional database of 4D flow MRI data was queried to identify a patient cohort with suspected isolated aortopathy and tricuspid aortic valve, and to assemble a comparison healthy subject cohort without disease. Patients with aortic valve stenosis (mild to severe), ejection fraction lower than 50%, history of aortic dissection, or history of valve / aortic replacement / repair were excluded. Healthy volunteer subjects were 18 years or older with no known cardiovascular disease. All subjects were included in this study with IRB supervision and approval.Imaging was performed using a time-resolved phase-contrast (4D flow) sequence at 1.5T and 3T with (1.2-3.1mm)3, 33-45ms resolution and coverage of the full thoracic aorta; other sequence parameters include tip angle 7-25°, 80-500cm/s VENC, 2.1-2.8ms TE, 4.1-5.7ms TR. Preprocessing to correct eddy current and concomitant gradient effects was performed, and the thoracic aorta was segmented with commercial image processing software.
Using a dimensionally-reduced model of aortic blood flow (figure 1), a fluid stability parameter Nω was derived from Navier-Stokes equations relating physical fluid pressure, cross-sectional area, and velocity to identify the threshold at which linear perturbations will cause unbounded growth to area 2,3. The pressure, area and velocity values used to compute Nω are taken directly or calculated from segmented aortic 4D flow MRI data (figure 1). Values of Nω above zero indicate theoretical fluid structure instability and expected potential for growth.
Chart reviews for patients were conducted to identify aortic measurements in all available clinical imaging (CT or MR) performed within 5 years after the date of the 4D flow MRI acquisition. Standardized assessments of sinus of valsalva (SOV) and maximal ascending aortic (MAA) diameters were extracted from the imaging reports, and interval growth events were identified as a change in maximal dimension between any successive time points above 3mm/year. Chart reviews also annotated aortic surgical interventions that involved replacement or repair of the aortic valve or ascending aorta.
In the patient cohort, the potential for Nω>0 to predict future adverse clinical events (interval growth/dilation or surgical intervention) was evaluated. Statistical comparisons for group differences used Wilcoxon rank sum tests, and true/false positives/negatives and receiver-operator curve (ROC) analysis were used to assess predictive performance.
Results
A total of 217 subjects were included, comprising 117 patients and 100 healthy subjects. In the patient cohort, 72 subjects had adequate follow-up data. Of those, 22 had interval growth >3mm in both/either MAA and/or SOV recorded at some time after the 4D flow MRI, and 6 subjects underwent aortic valve and/or wall repair (figure 3). The Nω>0 threshold for prediction of future adverse events achieved sensitivity/specificity of 0.91 and 0.98. Area under the curve from ROC analysis for Nω was 0.97 for predicting growth or surgical intervention within 5 years.The Nω values for patients (median: -1.24, IQR: [-2.43,1.04]) were generally higher than for healthy volunteers (med.: -2.26, IQR: [-3.13,-1.10]) with significance in Wilcoxon test (p<0.001) (figure 4). However, patients were not matched to the healthy subjects for age (46.4±15.5 years vs. 58.5±11.7 years) or sex (24% female vs. 51% female).
Discussion and Conclusion
The fluid-structure stability parameter, derived from physical first principles, is highly predictive of future aortic dilation and aortic surgery in a cohort of patients being imaged for suspected aortopathy. Comparison between patients and healthy subjects showed higher (less stable) values for the stability parameter in patients, but an age- and sex-matched comparison would be more conclusive for establishing group differences. Expanding the cohort size for the tricuspid aortic valve patients studied here and extending analysis to patients with bicuspid valves would help establish calculation of the fluid-structure as a valuable clinical metric for management of aortopathy.Acknowledgements
NIH R01HL115828, R01HL133504, TL1TR001423References
1. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease. Journal of the American College of Cardiology. 2021 Feb;77(4):e25–197.2. Azer K, Peskin CS. A One-dimensional Model of Blood Flow in Arteries with Friction and Convection Based on the Womersley Velocity Profile. Cardiovasc Eng. 2007 Jun 26;7(2):51–73.
3. Wang X-F, Nishi S, Matsukawa M, Ghigo A, Lagrée P-Y, Fullana J-M. Fluid friction and wall viscosity of the 1D blood flow model. Journal of Biomechanics. 2016 Feb;49(4):565–71.
Figures
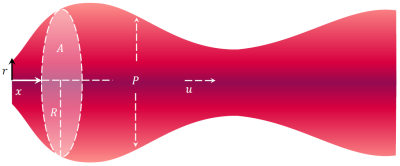
Figure 1. The aortic blood flow model used for analyzing 4D flow MRI data assumes movement of a fluid in a distensible tube with internal pressure $$$P$$$ and radially symmetric velocity component $$$u$$$ in the direction normal to the centerline length $$$x$$$. The cross-sectional area $$$A$$$ is modelled as both time- and space-varying.
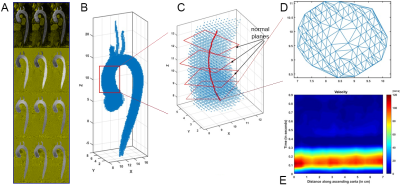
Figure 2. Processing of 4D flow MRI (A) for calculation of stability parameter Nω uses segmentation of the thoracic aorta (B) to place a centre-line and analysis planes through the ascending aorta (C), and generates cross-sectional meshing by Delaunay triangulation (D) to render a velocity map along the aortic length and through the cardiac cycle time (E).

Figure 3. Aortic growth/dilation rates (A) observed in follow-up data from the patient are labelled according to sign (positive/negative) for the stability parameter Nω and documented eventual surgical intervention. Prediction performance in terms of true/false positive/negative (B) for using Nω to future dilation and/or surgical repair of aortic valve/wall. Prediction of these outcomes from Nω achieves receiver operating characteristic area over 0.9 (C).
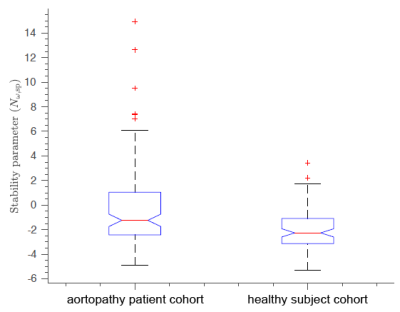
Figure 4. The stability parameter Nω is compared for suspected-aortopathy subjects and healthy subjects. The patient cohort parameter value is higher (less stable) than that of the healthy subject cohort (-1.2431 vs. -2.2648; p<0.001).
DOI: https://doi.org/10.58530/2022/0895