0894
Diffusion Tensor Imaging Helps to Differentiate Neuromyelitis Optica Spectrum Disorders and Multiple Sclerosis-Related Optic Neuritis1Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
The purpose of this study was to assess the differences in the patterns of NMOSD and MS-related optic nerve damage by DTI. In this study, the intraorbital optic nerve was divided anatomically in three equal parts. We found that NMOSD-related optic neuritis exhibited extensive optic nerve damage, particularly in the posterior segment of the optic nerve, whereas MS-related optic neuritis tended to be more anterior-middle optic nerve damage. In addition, the combination of FA with conventional MRI showed better differential diagnostic efficacy for NMOSD and MS-related optic neuritis.
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) and multiple sclerosis (MS) are both common demyelinating diseases of central nervous system and share similar symptoms of optic neuritis[1]. But treatment strategies have suggested to be utilized in clinics. MS treatment regimens may worsen NMOSD, so it is crucial to differentiate between the two in clinical practice[2; 3]. Diffusion tensor imaging (DTI) can quantitatively evaluate the integrity of white matter fiber bundles given water movement in random distribution. In this study, factional anisotropy (FA) was computed to assess the optic nerve impairment in NMOSD and MS and investigate the feasibility of FA to differentiate between the two diseases.Materials and methods
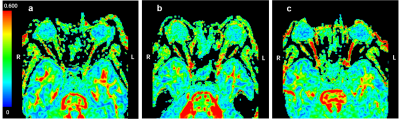
31 patients with NMOSD and 25 patients with MS were recruited. NMOSD and MS patients were divided into vision-impaired (VI) subgroup and normal-appearing (NA) subgroup according to their visual status, respectively. 17 age- and gender-matched healthy controls (HC) were included in the study. All subjects underwent MR imaging on a 3T MR scanner (Discovery MR750, GE Medical Systems) with a 32-channel head coil. The sagittal CUBE T2WI of head was obtained with the following parameters: TR/TE = 2500/84 ms, NEX = 1, matrix =256×256, slice thickness = 1 mm, slice spacing = 1 mm, FOV = 256×256 mm2, and acquisition time was 3 minutes 31 s. The axial optic nerve DTI was obtained parallel to the bilateral optic nerve by using spin-echo echo-planar imaging sequence with 64 non-collinear spatial directions and the scanning range was from the inferior to superior orbital rim. For DTI acquisitions, TR/TE = 2000/67 ms, NEX = 1, matrix = 128×128, slice thickness = 2 mm, slice spacing = 0 mm, 16 slices, FOV = 256×256 mm2, b = 0, 1000 s/mm2, and acquisition time was 2 minutes 12 s. The Fractional anisotropy (FA) maps were generated on GE Advantage workstation (version 4.5) from raw DTI data. FA values were measured in the anterior, middle, and posterior parts of each intraorbital optic nerve segment using ITK-SNAP software (version 3.8.0). The signal intensity ratio (SIR) on T2WI was defined as the optic nerve signal intensity divided by the brain white matter signal intensity. All statistical analyses were performed by using SPSS (Version 26.0.0, IBM) and MedCalc (Version 15.8, MedCalc Software). P < 0.05 was considered statistically significant.Results
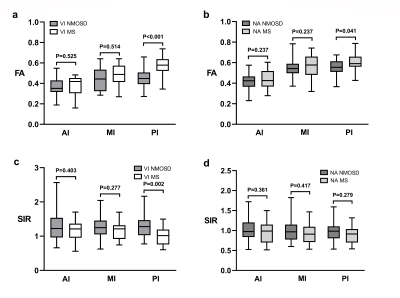
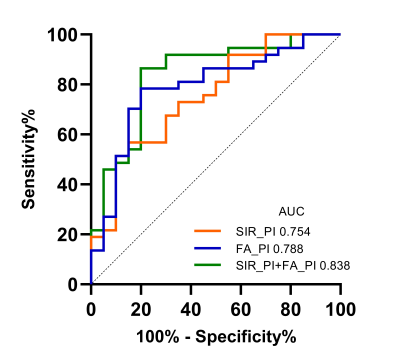
FA values in VI NMOSD significantly decreased in the whole optic nerve than those in HC, especially the posterior segment of the optic nerve (P<0.001) (Fig.1). FA values measured in the anterior and middle segments of optic nerve in VI MS were significantly decreased compared to HC (P<0.05). Between NMOSD and MS, FA values in the posterior segment of the optic nerve showed significant differences (VI NMOSD vs VI MS P<0.001; NA NMOSD vs NA MS, P=0.041, respectively) (Fig. 2). The signal intensity ratio (SIR) in the posterior segment of optic nerve significantly increased in VI NMOSD compared to VI MS (P=0.002). The combination of SIR and FA for distinguishing VI NMOSD from VI MS resulted in better sensitivity, specificity, positive and negative predictive values of 86.49 %, 80.00 %, 88.9 % and 76.2 %, respectively (Fig. 3).Discussion
Our study found that FA values, particularly measured in the posterior segment of the optic nerve, can help to distinguish the different patterns of optic nerve damage between NMOSD and MS. VI NMOSD appeared a wide range of optic nerve impairment, from the anterior to the posterior segment of the optic nerve, but the posterior segment of the optic nerve is more commonly involved[4]. The impairment of the optic nerve in MS was relatively involved in short segment [5]. FA values significantly decreased in the anterior and middle segments of the optic nerve in VI MS patients. We speculated that NMOSD caused more severe optic nerve damage than MS, with more pronounced changes in optic nerve fiber structure and more aggressive demyelination, leading to a widespread decrease in FA. These findings can be explained by the results of previous studies that Papadopoulos et al [6] found the NMOSD-related optic neuritis differs from the acute demyelination of MS-related optic neuritis in that the inflammatory infiltration and necrosis produced by AQP4 antibodies resulted in more severe axonal damage. Abnormal T2 signal of the optic nerve is one of the current diagnostic criteria for optic neuritis; however, image quality and sensitivity of results were easily affected by environment, equipment, technique, patient factors such as layer thickness and eye movement. FA was sensitive in detecting abnormalities in optic nerve integrity caused by optic nerve damage. The combination of FA and SIR on T2WI allowed for more sensitive and accurate diagnosis of the severity and anatomical site of optic nerve damage, facilitating the differentiation between NMOSD and MS.Conclusion
NMOSD and MS-related optic nerve impairment cause to decreased FA values in different segments of the optic nerve. DTI may be a simple and effective imaging tool to assess NMOSD and MS-related optic nerve impairment.Acknowledgements
Funding: This project was supported by the National Natural Science Funds of China (Grants No.81730049).References
1. Liu Y, Dong D, Zhang L et al (2019) Radiomics in multiple sclerosis and neuromyelitis optica spectrum disorder. Eur Radiol 29:4670-4677
2. Palace J, Leite MI, Nairne A, Vincent A (2010) Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol 67:1016-1017
3. Min JH, Kim BJ, Lee KH (2012) Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 18:113-115
4. Chen Z, Lou X, Liu M et al (2015) Assessment of Optic Nerve Impairment in Patients with Neuromyelitis Optica by MR Diffusion Tensor Imaging. PLoS One 10:e0126574
5. Dutra BG, da Rocha AJ, Nunes RH, Maia ACMJ (2018) Neuromyelitis Optica Spectrum Disorders: Spectrum of MR Imaging Findings and Their Differential Diagnosis. Radiographics 38:169-193
6. Papadopoulos MC, Verkman AS (2012) Aquaporin 4 and neuromyelitis optica. The Lancet Neurology 11:535-544
Figures