0880
A new framework to build high quality tractography-based DWI brain templates using COMMIT1Natbrainlab, King's College London, London, United Kingdom, 2University College London, London, United Kingdom, 3Universita di Verona, Verona, Italy
Synopsis
In this study we present a novel method for building high quality tractography-based DWI brain templates. We use streamline normalisation to obtain the correct anatomical fibre orientation information in template space and then regenerate the diffusion signal using the COMMIT framework. The template fODF field and tractography reconstructions show good anatomical agreement with well known white matter structures. This framework could provide new templates, atlases and tools for normalisation of DWI data, and simplify the creation of realistic DWI phantoms.
Introduction
Brain templates and atlases are vital tools in neuroimaging research. They can define reference spaces for normalisation of brain images, allowing comparison of equivalent anatomical locations between individuals or across imaging modalities1. They can also be used to create group level models for the study of brain development and pathology2, or for automated parcellation of brain structures3.Creating brain templates from diffusion MRI images poses a particular problem since they contain directional information reflecting complex fibre configurations at each voxel which needs to be properly handled. Several approaches to this problem have already been proposed which involve calculating transforms at the level of the diffusion tensor or fibre orientation distribution function (fODF) which are used for normalisation to a target space3,4,5.
In this work we introduce a novel method for DWI template construction by applying transformations to whole brain tractograms to preserve the correct anatomical fibre orientations in target space. We leverage the COMMIT framework6 to identify the best white matter signal representation based on the tractogram in each subject’s native space and regenerate the final signal in MNI space.
Methods
Multi-shell diffusion MR images from 20 subjects scanned as part of the Human Connectome Project7 were used in this study. Damped Richardson-Lucy spherical deconvolution8 was applied and deterministic whole brain tractography was generated for each subject’s native space using StarTrack9.The template construction consists of three main steps:
- Using the COMMIT framework6, a multi-compartmental model was fitted to compute the global signal contribution of every streamline in order to explain the native space diffusion signal of each subject. Each voxel contains intra-cellular (IC) compartments oriented along each streamline, and two separate isotropic compartments for grey matter and CSF. Streamlines that were not compatible with the acquired signal were filtered out.
- Streamlines and compartment maps were then nonlinearly warped to MNI space using precomputed transformation fields following the Megatrack framework10.
- Finally, the diffusion signal of each subject was regenerated in MNI space using the individual contributions associated with each streamline and isotropic volume fractions before being averaged across all subjects.
Results
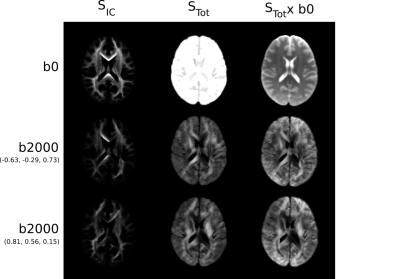
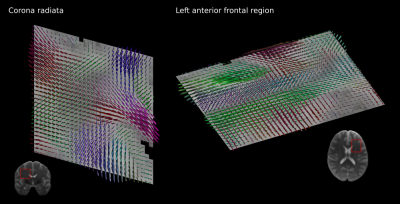
The reconstructed average diffusion signal in MNI space for two diffusion gradient directions is displayed in Figure 1. The group averaged intra-cellular (IC) contributions to the signal are shown separately to the total signal to emphasise the preservation of anisotropy in the reconstruction.Template fODF fields in a coronal slice through the corona radiata and an axial slice of the left anterior frontal lobe are shown in Figure 2. The regenerated DWI signal reproduces highly detailed fODFs showing complex white matter organisation including sharp crossings and smaller white matter components while providing a smooth representation of the DWI signal in MNI space.
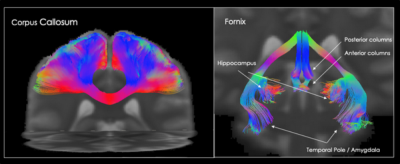
Finally, tractography obtained from the average MNI diffusion signal is shown in Figure 3. On the left, the dissection of the central portion of the corpus callosum reveals a complete reconstruction of the commissural tract showing no gaps or missing connections between the two hemispheres. Streamlines follow their expected trajectory to the cortex with very low noise or jitter in their propagation. A reconstruction of the fornix also shows a high level of anatomical detail. This includes a bilateral reconstruction of multiple dense terminations in both the hippocampal and the anterior temporal regions reaching the amygdala, which are not always visible in single subject dissections.
Discussion
In this study we have presented a novel method for building high quality tractography-based DWI brain templates. We regenerate the diffusion signal from tractography in MNI space using the COMMIT framework, and subsequently obtain a group averaged DWI signal template. The average fODF fields and tractography reconstructions showed sharp fibre orientations and fine anatomical detail in close agreement with well known white matter structures. By directly mapping streamlines into the target space we were able to preserve the correct anatomical orientation of different fibre populations within each voxel without introducing blurring in the fODFs or affecting other fibre populations in the same voxel.Since the regenerated white matter signal can be kept separate from the other diffusion components, this framework can also be used to construct DWI phantoms to test new diffusion models against realistic simulations. The template thus provides a gold standard reference for the underlying white matter orientation, volume fractions and diffusivities of the different compartments.
The quality of the final template can be further improved by applying more advanced frameworks like COMMIT211 and more complex diffusion models to better characterize the original signal. Future work will investigate different options to identify the optimal pipeline, increase the number of subjects and will compare the results with templates built using alternative methods.
Conclusion
In summary, we have developed a method for creation of high quality tractography-based DWI human brain templates using COMMIT and streamline normalisation to preserve the correct fibre orientation information in the target space. Our results show good anatomical agreement with known white matter structures and could provide a new tool for normalisation of complex diffusion data and creation of advanced realistic DWI phantoms.Acknowledgements
No acknowledgement found.References
1. Talairach J and Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical New York 1988.
2. Maziotta J, Toga A, Evans A et al. A Four-Dimensional Probabilistic Atlas of the Human Brain. J Am Med Inform Assoc. 2001; 8(5):401–430.
3. Mori S, Oishi K, Jiang H et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 2008; 40(2):570-582.
4. Raffelt D, Tournier D, Fripp J et al. Symmetric diffeomorphic registration of fibre orientation distributions. NeuroImage 2011; 56(3):1171-1180.
5. Varentsova A, Zhang S, Arfanakis K. Development of a high angular resolution diffusion imaging human brain template. NeuroImage 2014; 91:177-186.
6. Daducci A, Dal Palu A, Lemkaddem A et al. COMMIT: Convex Optimization Modeling for Microstructure Informed Tractography. IEEE Transactions on Medical Imaging 2015; 34(1):246-257.
7. Van Essen D, Smith S, Barch D et al. The WU-Minn Human Connectome Project: An overview. NeuroImage 2013. 80:62-79.
8. Dell'Acqua F, Scifo P, Rizzo G et al. A modified damped Richardson-Lucy algorithm to reduce isotropic background effects in spherical deconvolution. NeuroImage 2010; 49(2):1446-1458.
9. www.mr-startrack.com
10. Dell’Acqua F, Lacerda L, Barrett R et al. Megatrack: A fast and effective strategy for group comparison and supervised analysis of large-scale tractography datasets. Proc. Intl. Soc. Mag. Reson. Med. 2015.
11. Schiavi S, Ocampo-Pineda M, Barakovic M et al. A new method for accurate in vivo mapping of human brain connections using microstructural and anatomical information. Science Advances 2020; 6(31).
Figures