0876
Resolving heterogeneous crossing fibers with Adaptive modelling and Generalized Richardson Lucy spherical deconvolution (AGRL)1Neurology Department, UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht, Netherlands, 2Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 3Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 4Department of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States
Synopsis
We evaluate the benefits of shifting from a global white matter (WM) model to an adaptive (voxel-wise) model in the Generalized Richardson Lucy (GRL) framework. Using simulations, we show that GRL with an adaptive model (AGRL) could resolve crossing fiber configurations with heterogeneous properties, whereas conventional GRL did not. In in-vivo data, AGRL simultaneously used different deconvolution models. Compared to GRL, fiber orientation distributions of AGRL showed remarkable angular differences, especially for the second and third peak. Tractography with AGRL resulted in a more extensive reconstruction of the arcuate fasciculus, suggesting adaptive modelling as a promising future direction.
Introduction
Spherical deconvolution methods1,2 leverage high-angular resolution brain diffusion MRI (dMRI) data to determine the orientation of crossing fibers in white matter (WM). Most spherical deconvolution methods use a global model of single-fiber WM (i.e., response function) across the brain to determine the fiber orientation distribution (FOD), implicitly assuming WM to be homogeneous. However, different WM fibers can exhibit heterogeneous properties3–5. In this work, we extend a recently introduced model-based spherical deconvolution framework6 to account for heterogeneous WM properties, and show proof-of-concept of its performance with synthetic and in-vivo MRI data.Methods
Adaptive spherical deconvolutionThe starting point of this work is the Generalized Richardson Lucy (GRL) deconvolution framework6. In GRL, we aim to solve the signal (S) equation $$$S=K*FOD$$$. The columns of the deconvolution matrix K contain possible solutions to the deconvolution problem, e.g., projections of a single-fiber WM model along several directions uniformly distributed on the unit sphere. Instead of using a single K, with Adaptive GRL (AGRL) we propose to account for heterogeneous WM properties by generating N instances of K (Ki) corresponding to different parametrizations of a chosen signal model. In a first iteration, GRL is repeatedly performed for each Ki, and the columns of Ki that do not contribute to the FOD are removed. In a second iteration, the retained columns of all Ki are joined in a final deconvolution matrix K, and GRL is applied one final time to determine the final FOD.
Experiments
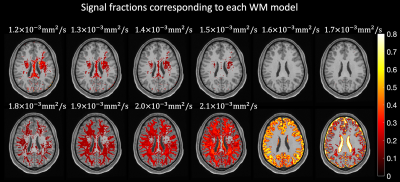
In all experiments, we used the diffusion tensor imaging (DTI) model to simulate signals. Ten possible Ki were generated by varying the axial diffusivity in 10 steps between 1.2x10-3mm2/s and 2.1x10-3mm2/s while keeping the radial diffusivity fixed to a value estimated per dataset as previously described6.
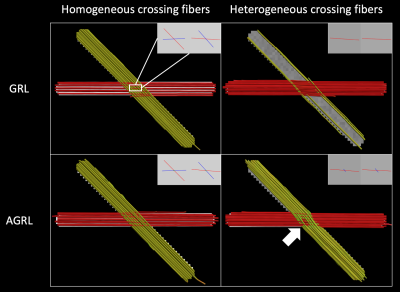
Experiment I: two crossing fibers (angle 45°) were generated with homogeneous (identical fractional anisotropy and mean diffusivity) and heterogeneous WM properties (different fractional anisotropy), respectively, with signal to noise ratio (SNR) at b = 0 s/mm2 equal to 30 using ExploreDTI7. The FOD was determined with GRL and AGRL, then deterministic fiber tractography was performed with step size 1mm, angle threshold 30°.
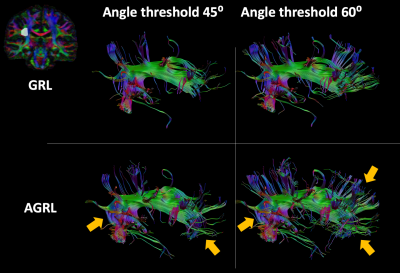
Experiment II: We compared AGRL to GRL using 1 dataset from the Human Connectome Project8. We evaluated the spatial distribution of the signal fractions associated to each of the 10 WM-models, then evaluated the prevalence of each WM-model in the whole WM mask (segmented from a co-registered T1-weighted9). Subsequently, the peaks associated to each FOD were extracted10 to determine their orientation and amplitude. Peaks with amplitude below 0.05 were discarded. Finally, fiber tractography of the arcuate fasciculus was performed with both GRL and AGRL using a deterministic approach with step-size 0.6mm, angle threshold 45° and 60°.
Results
Experiment IResults in Fig. 1 show that GRL could only resolve homogeneous crossing fibers. Conversely, AGRL correctly reconstructed both homogeneous and heterogeneous configurations, albeit with a small angular error (white arrow, Fig. 1). This results suggest that a global WM model leads to erroneous FOD estimation when it does not match the underlying tissue properties, as expected3.
Experiment II
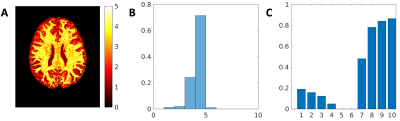
The signal fractions associated to each Ki when applying AGRL to in-vivo data are shown in Fig. 2. Part of the corpus callosum and of the deep WM are better described by Ki with lower axial diffusivity (corresponding to about 20% of the WM, Fig. 3C), whereas most superficial WM is better described by a Ki with higher axial diffusivity (~80% of WM, Fig. 3C). In most WM voxels, 3 to 4 WM-models are simultaneously used by AGRL (Fig. 3A-B).
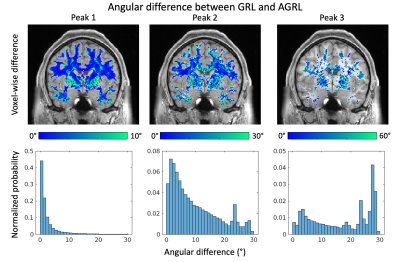
When comparing at the first FOD peak estimated with GRL and AGRL (Fig. 4), we observed little angular differences in deep WM. However, differences above 10° were observed for the first peak in regions with 3+ crossing fibers such as the centrum semiovale, and in proximity of (deep) gray matter. In over 10% of WM, angular differences for the second and third peaks were larger than 10°.
In Fig. 5, we evaluated the impact of adaptive modelling fiber tractography of the arcuate fasciculus. In general, tractography with AGRL resulted in longer projections in the anterior part of the tract, larger coverage of the frontal lobe, and a more consistent representation of the bending part of the tract (see orange arrows in Fig. 5). These results were observed for both tested angular thresholds.
Discussion and conclusions
In this work, we have shown that using a global WM-model can lead to failures at detecting crossing fiber configurations when the underlying WM properties are largely different from those of the employed model. When applying AGRL in in-vivo data, we observe that multiple WM-models are simultaneously used in most WM voxels (>90%). While recent work suggests a global WM-model is sufficient to perform spherical deconvolution11, we observed considerable angular differences between GRL and AGRL. Angular differences are more apparent in the second and third peak than in the first peak and might thus be unobserved if not using high-quality dMRI that allows to disentangle 2+ peaks robustly. Altogether, our results suggest that a spatially adaptive WM-model might improve the FOD estimation, and be beneficial for fiber tractography of the arcuate fasciculus and likely other tracts.Acknowledgements
ADL and GJB are supported by a VICI grant 918.16.616 from ZonMw, The Netherlands, Organisation for Health Research and Development (PI, GJ Biessels).
KS is supported by the National Institutes of Health under award numbers R01EB017230, and T32EB001628, and in part by ViSE/VICTR VR3029 and the National Center for Research Resources, Grant UL1 RR024975-01.
CMWT was suppported by a Veni grant (17331) from the Dutch Research Council (NWO), and a Sir Henry Wellcome Fellowship (215944/Z/19/Z)
References
1. Tournier, J. D., Calamante, F. & Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35, 1459–1472 (2007).
2. Dell’Acqua, F. et al. A model-based deconvolution approach to solve fiber crossing in diffusion-weighted MR imaging. IEEE Trans. Biomed. Eng. 54, 462–72 (2007).
3. Guo, F. et al. Fiber orientation distribution from diffusion MRI: Effects of inaccurate response function calibration. J. Neuroimaging 1–17 (2021) doi:10.1111/jon.12901.
4. Schilling, K. G., Landman, B. A., Leemans, A., Anderson, A. W. & Tax, C. M. Diffusion MRI response function estimates vary more across pathways than across subjects. in International Society for Magnetic Resonance in Medicine (2020).
5. de Almeida Martins, J. P. et al. Computing and visualising intra-voxel orientation-specific relaxation–diffusion features in the human brain. Hum. Brain Mapp. 42, 310–328 (2021).
6. Guo, F., Leemans, A., Viergever, M. A., Dell’Acqua, F. & De Luca, A. Generalized Richardson-Lucy (GRL) for analyzing multi-shell diffusion MRI data. Neuroimage 218, 116948 (2020).
7. Leemans, A., Jeurissen, B., Sijbers, J. & Jones, D. K. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. in 17th annual meeting of the International Society for Magnetic Resonance in Medicine, Honolulu, Hawaii, USA 3537 (2009).
8. McNab, J. A. et al. The Human Connectome Project and beyond: initial applications of 300 mT/m gradients. Neuroimage 80, 234–45 (2013).
9. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–90 (2012).
10. Jeurissen, B., Leemans, A., Tournier, J.-D., Jones, D. K. & Sijbers, J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 34, 2747–66 (2013).
11. Christiaens, D. et al. On the need for bundle-specific microstructure kernels in diffusion MRI. Neuroimage 208, 116460 (2020).
Figures