0872
On the anisotropy of IVIM-derived microvascular cerebral pseudo-diffusion: a physics-informed neural network approach1Department of Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2School for Mental Health & Neuroscience, Maastricht University, Maastricht, Netherlands, 3Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 4Department of Neurology, Maastricht University Medical Center, Maastricht, Netherlands, 5School for Cardiovascular Disease, Maastricht University, Maastricht, Netherlands
Synopsis
Acquisition of intravoxel incoherent motion (IVIM) images with diffusion sensitization in at least six directions (IVIM tensor imaging) provides the unique opportunity to non-invasively measure the anisotropy of both the parenchymal and microvascular diffusivity (D and D*). We demonstrate the feasibility of whole-brain IVIM tensor analysis by utilizing a physics-informed neural network fitting approach to achieve more accurate assessment of D, D*, and the corresponding tensors. The fractional anisotropy of D* (FA(D*)) was explored for different brain tissue regions, which revealed lower FA(D*) in cortical gray matter and higher FA(D*) in deep gray matter compared to white matter.
Introduction
Intravoxel incoherent motion (IVIM) MRI is a diffusion-weighted technique that is sensitive to diffusion of water in the parenchyma (D), as well as flow-mediated diffusivity of microvascular blood (D*).1 This technique is relevant for research on pathologies in which both the microstructural integrity and microvasculature are altered, e.g. cerebral small vessel disease (cSVD).2 To estimate the microvascular component, the IVIM signal attenuation is sampled across a range of diffusion sensitizing b-values, and can be decomposed (for a given direction) into a parenchymal and a microvascular component by fitting a bi-exponential model.1 When the diffusion sensitization is applied in at least six directions, the anisotropy of diffusing water molecules can be characterized, which is a well-established method (DTI; diffusion tensor imaging) to measure the direction of large axon bundles using the D-tensor.3The combination of IVIM and DTI has the potential to estimate the anisotropy of microvascular pseudo-diffusion, which could reveal unique information on the preferred orientation of the functional microvascular architecture. However, previous studies that employed IVIM tensor imaging, reported that estimating the D*-tensor was challenging since the fast signal decay part pertaining to D* is small and strongly suffers from image noise.4,5 Fitting the bi-exponential IVIM model using a recently developed physics-informed neural network (PI-NN), was shown to provide a more robust estimation of D* compared to conventional fitting methods6, and might yield a more accurate estimation of the D*-tensor. Therefore, we utilize this state-of-the-art fitting method to explore the feasibility of whole-brain IVIM tensor imaging and simultaneously examine the potential anisotropic behaviour of D* over different brain tissue regions.
Methods
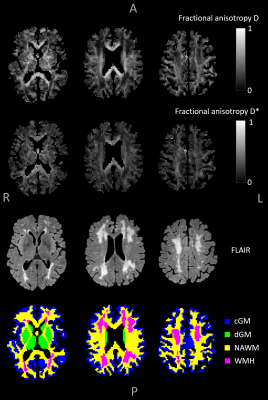
MRI acquisition: Eleven participants (seven patients with vascular cognitive impairment due to cSVD and four cognitively normal subjects) underwent whole-brain imaging (3T MRI, Philips, Achieva TX). IVIM tensor imaging was acquired with fourteen non-zero diffusion sensitive b-values in six noncollinear gradient directions, and included cerebrospinal fluid suppression (single-shot spin-echo echo-planar-imaging (EPI), 2.4 mm cubic voxel size, b-values: 0,10,20,30,40,50,60,100,200,300,400,500,600,800,1000 s/mm2). An additional b=0-image with reversed phase-encoding direction was acquired for distortion correction. Furthermore, T2-weighted FLAIR and T1-weighted images were acquired for segmentation of different brain regions of interest (ROIs).Image analysis: The IVIM images were corrected for geometric EPI distortions (topup, FSL) and head displacements (ExploreDTI). Cortical gray matter (cGM), deep gray matter (dGM) and white matter (WM) were automatically segmented (Freesurfer), whereas white matter hyperintensities (WMH) were manually delineated. The bi-exponential IVIM model was fitted to the signal decay curves in a voxel-wise manner by means of a PI-NN.6 Briefly, the PI-NN uses a fully-connected 2-layer neural network for each IVIM parameter and it learns the physics of the IVIM model due to the implementation of the IVIM signal decay formula in the loss-function.6
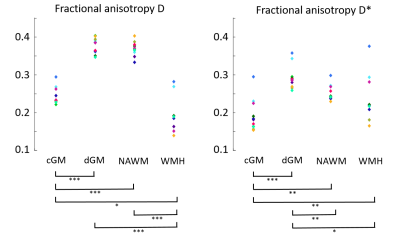
Tensor calculation: The IVIM metrics were calculated for each of the six gradient directions. Subsequently, the six independent tensor elements were calculated by the matrix product of the pseudo-inversed B-matrix and the estimated diffusivities (D and D*).5 Next, the tensor was quantified by computing the eigenvalues. From these eigenvalues, the fractional anisotropy (FA) was calculated for both D and D*.7 Median FA values were obtained in the cGM, dGM, normal-appearing WM (NAWM) and WMH.
Statistical analysis: Within-subjects ANOVA with post-hoc analyses and Bonferroni corrections were conducted to identify significant differences across the ROIs for FA(D*) and FA(D).
Results
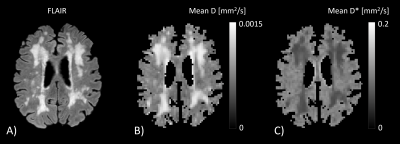
Figure 1 visualizes the mean D- and D*-maps and Figure 2 shows FA(D)- and FA(D*)-maps of a patient with vascular cognitive impairment. The large fiber bundles are clearly enhanced on the FA(D)-map, and the FA(D*)-map follows the same organization as FA(D), although with lower values. Figure 3 plots the median FA(D*) and FA(D) for cGM, dGM, NAWM and WMH. Two participants had a low WMH load (<3.0 mL), hence the FAs in WMH of these participants were excluded from analysis.The within-subjects ANOVA revealed significant differences of FA(D*) between the tissue types (F=36.38, p<.001). Post-hoc analysis showed that the FA(D*) of cGM was lower compared to dGM (p<.001), NAWM (p=.001) and WMH (p=.001), and that the FA(D*) of dGM was higher compared to NAWM (p=.001) and WMH (p=.019). FA(D) was also found to be different across the tissue types (F=71.85, p<.001). The post-hoc test showed lower FA(D) in WMH compared to cGM (p=.027), dGM (p<.001) and NAWM (p<.001), and lower FA(D) in cGM compared to dGM and NAWM (both p<.001).
Discussion & Conclusion
The current study demonstrated the feasibility of whole-brain IVIM tensor imaging in combination with a PI-NN for characterising the orientation of the microcirculatory network. The in vivo results were in line with a prior post-mortem human histology study, where the FA of microvessels was found to be lower in the cGM compared to the WM and dGM.8 Furthermore, the mean diffusivity maps (D and D*) were consistent with biological expectations2,11, and our measured FA(D) of the ROIs was in agreement with previous DTI studies.9,10 Compared to conventional DTI, IVIM tensor imaging might provide more accurate estimation of the parenchymal diffusion, as it adjusts for additional phase incoherence effects due to the microcirculatory anisotropy. This study opens up new possibilities to gain insight in the preferred orientation of functional microvascular networks and its alteration, for instance due to pathology.Acknowledgements
This work has received funding from the European Union’s Horizon 2020 research and innovation programme ‘CRUCIAL’ under grant number 848109.References
1. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401-7.
2. Wong SM, Zhang CE, van Bussel FC, Staals J, Jeukens CR, Hofman PA, et al. Simultaneous investigation of microvasculature and parenchyma in cerebral small vessel disease using intravoxel incoherent motion imaging. NeuroImage: Clinical. 2017;14:216-21.
3. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316-29.
4. Zhang XS, Sang XQ, Kuai ZX, Zhang HX, Lou J, Lu Q, et al. Investigation of intravoxel incoherent motion tensor imaging for the characterization of the in vivo human heart. Magnetic Resonance in Medicine. 2021;85(3):1414-26.
5. Finkenstaedt T, Klarhoefer M, Eberhardt C, Becker AS, Andreisek G, Boss A, et al. The IVIM signal in the healthy cerebral gray matter: a play of spherical and non-spherical components. Neuroimage. 2017;152:340-7.
6. Kaandorp MP, Barbieri S, Klaassen R, van Laarhoven HW, Crezee H, While PT, et al. Improved unsupervised physics‐informed deep learning for intravoxel incoherent motion modeling and evaluation in pancreatic cancer patients. Magnetic Resonance in Medicine. 2021;86(4):2250-65.
7. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209-19.
8. Kochová P, Cimrman R, Janáček J, Witter K, Tonar Z. How to asses, visualize and compare the anisotropy of linear structures reconstructed from optical sections—A study based on histopathological quantification of human brain microvessels. Journal of theoretical biology. 2011;286:67-78.
9. Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O’Brien JT, et al. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clinical science. 2017;131(12):1361-73.
10. Fox R, Sakaie K, Lee J-C, Debbins J, Liu Y, Arnold D, et al. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. American Journal of Neuroradiology. 2012;33(4):695-700.
11. Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. Frontiers in neurology. 2021;12:621.
Figures