0869
VERDICT MRI for tumor tissue characterization – separating viable tumor from necrosis1Medical radiation sciences, Institute of Clinical Sciences, University of Gothenburg, Gothenburg, Sweden, 2Medical Physics and biomedical engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
Synopsis
The biological meaning of MRI-based biomarkers of cancer can be studied using spatially matched histologically stained tumor sections. However, correlations are often weak or contradictory, possibly due to confounding necrosis that models fail to describe. In this work, we demonstrate the potential of using VERDICT MRI model parameters to separate necrosis from viable tumor tissue non-invasively. Radiation treated tumors demonstrating large necrotic and viable regions are imaged, excised and stained using immunohistochemical methods. Correlations between spatially matched VERDICT and immunohistochemical maps reveal that VERDICT clearly separates necrosis from viable tumor.
Introduction
Methods for non-invasive tumor tissue characterization would facilitate personalized treatment strategies and longitudinal response assessment for early adjustments of inefficient treatment. The literature suggests that histological features can be interrogated and visualized using MRI methods. However, biological validation of MRI-derived model parameters based on spatially matched histological data often produce varying and contradictory results, possibly due to inclusion of tissue types that are not included in the current models, such as non-cellular necrotic remnants and scar tissue1. In this work, we investigate the possibility to use parameters derived from the vascular, extracellular and restricted diffusion for cytometry in tumors (VERDICT) model2 to non-invasively separate necrotic- from viable tumor regions.Methods
Female nude mice (n=4) with subcutaneous human neuroendocrine tumors (GOT1) were imaged 15 days after single-fraction external radiation treatment of 8 Gy to the tumor (6 MV photons, Varian Medical Systems), to obtain large regions of necrosis, while other regions contain re-growing viable tumor.The study was approved by the Gothenburg Ethical Committee on Animal Research
Anesthetized (isoflurane, Isoba vet., Denmark) animals were imaged using a preclinical 7T MR system (Bruker BioSpin MRI GmbH, Ettlingen, Germany; software: ParaVision 5.1), including diffusion weighted imaging (DWI, spin-echo echo-planar imaging: 15 b-values: 28-3650 s/mm2, gradient duration/separation: 4-10/9-40 ms, echo time (TE): 19-56 ms) optimized for VERDICT and conventional DWI models (not shown)2,3. The minimum TE for each diffusion time and gradient duration combination was used to maximize signal-to-noise ratio. T2 effects were compensated for by normalizing to the b=0 images for image sets of identical TE. Pixel size: 400×400 μm2, slice thickness/gap: 0.5/0.5 mm, repetition time: 3000 ms, averages: 20, partial Fourier acceleration: 1.5. DWI scan time: ~50 min.
The AMICO framework4 was used to estimate fractional intracellular (fIC), extracellular extravascular (fEES) and vascular (fVASC) spaces, and cell radii (R) from VERDICT data.
Immediately after imaging, tumors were removed and processed as described elsewhere5 to enable spatial matching between MR and histology data. Hematoxylin/Eosin (HE), Ki67, VEGF, aSMA, CD31 and Masson Trichrome (MT), were used to reveal tumor cell morphology, proliferation, angiogenic activity, vessel maturation, vessel presence and fibrosis/scar, respectively.
The HALO pathology software (Indica labs, New Mexico, USA) was used to segment positive stain from surrounding tissue.
Three markedly different tissue types denoted “viable” (high tumor cell count), “necrotic” (lack of tumor cells, eosin stained) and “other” (mixture of tissue types) were identified on HE images, (Fig.1), in which 20 regions-of-interest (ROIs) (5 viable, 4 necrotic, 11 other) were drawn where they could also be clearly identified on DWIs. The area fraction of positively stained tissue was calculated for each ROI and stain and compared with corresponding VERDICT data.
Statistical methods used were the Pearson linear correlation coefficient and 2-sample t-tests.
Results
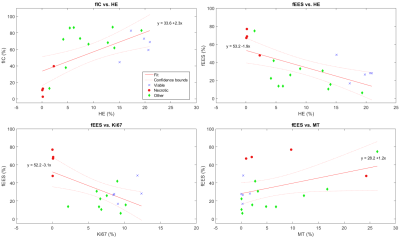
Analyzes of the different types of staining in the three ROI types (viable, necrotic and other) in the histological images yielded significantly different Fractional Area of Positively stained tissue (fAp) for HE and Ki67 (Fig.1&2). fAp of VEGF and MT were not statistically different, but indicated less angiogenic activity and connective tissue in the necrotic regions compared to the viable regions, whereas smaller differences were found for aSMA and CD31 between regions (Fig.2)There was a positive correlation between fIC and HE (p=0.0031), and fIC also demonstrated a clear separation between necrotic and viable tissue regions (Fig.3). The opposite correlation was found between fEES and HE (p=0.0025) although with slightly less separation between viable and necrotic areas (some overlap on y-axis). fEES also correlated negatively with the Ki67 representation of tissue types (p=0.009) and positively with the MR representation, although statistical significance was not reached (p=0.06). We found no correlations between R or fVASC and histological stain data.
Discussion
In this study, we have shown that it seems possible to use VERDICT data to separate necrotic from viable cell-dense or mixed heterogeneous tumor tissue. Both fIC and fEES showed significant correlations with area fraction of HE and Ki67 staining, and clusters of data from necrosis ROIs were well separated from other tissue types (Fig3.). It should be noted, however, that fIC and fEES have an almost inverse correlation, which only differs on the relative size of the vascular compartment (fVASC). The choice of parameter to use for necrosis determination may therefore need further evaluation.The ROI positions on histology were manually copied to the DWIs by careful selection of tumor landmarks. Although this approach is somewhat subjective, alternatives based on complex image registration techniques have proven difficult due to the multiple sources of tissue distortions associated with MRI and histological preparation1.
Conclusion
The VERDICT derived fraction of intracellular or extracellular extravascular space show promise as imaging biomarkers for separating necrotic from other tumor tissue types, and should be further evaluated as a means to improve biological validation of MRI biomarkers.Acknowledgements
No acknowledgement found.References
1. Alyami W, Kyme A, Bourne R. Histological Validation of MRI: A Review of Challenges in Registration of Imaging and Whole-Mount Histopathology. J Magn Reson Imaging. 2020. doi:10.1002/JMRI.27409
2. Panagiotaki E, Walker-Samuel S, Siow B, et al. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res. 2014;74(7):1902-1912. doi:10.1158/0008-5472.CAN-13-2511
3. Jalnefjord O, Montelius M, Starck G, Ljungberg M. Optimization of b-value schemes for estimation of the diffusion coefficient and the perfusion fraction with segmented intravoxel incoherent motion model fitting. Magn Reson Med. 2019;82(4). doi:10.1002/mrm.27826
4. Bonet-Carne E, Johnston E, Daducci A, et al. VERDICT-AMICO: Ultrafast fitting algorithm for non-invasive prostate microstructure characterization. NMR Biomed. 2019;32(1). doi:10.1002/NBM.4019
5. Montelius M, Jalnefjord O, Spetz J, Nilsson O, Forssell-Aronsson E, Ljungberg M. Multiparametric MR for non-invasive evaluation of tumour tissue histological characteristics after radionuclide therapy. NMR Biomed. 2019;32(3). doi:10.1002/nbm.4060Figures
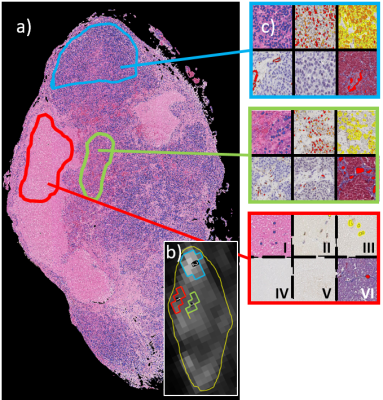
Figure 1. Hematoxylin & eosin stained tumor section (a) and corresponding VERDICT derived parametric map (b). The three regions marked in blue, red and green show typical ROIs used to extract features from viable, necrotic and other tumor tissue types, respectively. The magnified panels (c) show HE, Ki67, VEGF, aSMA, CD31 and MT stained tissue in position I)-VI), respectively, for the three tissue types, after segmentation (color overlays)
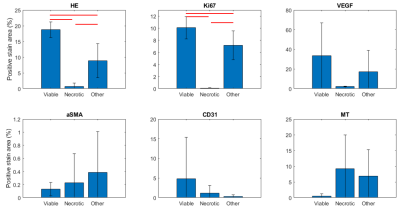
Figure 2. Mean (± SD) ROI fractional area of positively stained tissue for viable, necrotic and other tissues are shown for the different stains. Red bars indicate statistically significant difference at the 5% level (2-sample t-test). HE = hematoxylin & eosin, Ki67 = proliferation marker, VEGF = vascular endothelial growth factor, aSMA = alpha smooth muscle actin, CD31 = endothelial cell marker, MT = Masson Trichrome