0867
Comparison of image and extracorporeal derived arterial input functions for DCE-MRI in mice using an multimodal cross-validation approach1European Institute for Molecular Imaging, University of Münster, Münster, Germany, 2Translational Research Imaging Center, University of Münster, Münster, Germany, 3Institute of Inorganic and Analytical Chemistry, University of Münster, Münster, Germany, 4Department of Nuclear Medicine, University Hospital Münster, Münster, Germany
Synopsis
Quantitative precise measurements of the dynamic arterial blood concentration (AIF) are challenging in perfusion MRI. As solution an extracorporeal circulation approach for the AIF determination with two different vascular access ways is presented and cross validated with injection of radioactive contrast agent derivatives. Independent of the vascular access way, extracorporeal derived AIFs point towards a high quantitative precision, although dispersion must be considered. However, deconvolution methods based on single gamma variate functions end up in plausible AIFs. The use of novel rapid imaging techniques in connection with extracorporeal AIFs indicates high potential for precise quantitative dual PET/MRI Pharmacokinetic-Modeling in mice.
Introduction
Quantitative precise measurements of the dynamic arterial blood concentration (Arterial Input Function, AIF) are challenging in perfusion MRI, especially in small animals. However, quantitative precision of the AIF directly impacts parameters derived with pharmacokinetic modeling (PKM). In clinical and preclinical Positron Emission Tomography (PET), extracorporeal recordings of the AIF are common practice. We recently transferred this approach to small animal dynamic contrast-enhanced (DCE)-MRI and demonstrated successful recordings of the AIF in extracorporeally perfused blood reservoirs placed inside the MRI field of view 1. However, the introduced approach left open questions regarding the (a) ideal vascular access ways, (b) quantitative precision, (c) ideal dispersion correction and (d) the applicability with compressed sensing sequences. In the current study, we address these open questions by (a) comparing vascular access through the femoral artery and the carotid arteries, (b) validating the quantitative precision with simultaneous recordings of extracorporeal contrast agent radiotracer analogs and with inductively coupled plasma mass spectrometry (ICP-MS), (c) comparing two different dispersion correction methods and (d) applying state of the art compressed sensing and parallel imaging sequences for DCE and T1-mapping.Methods
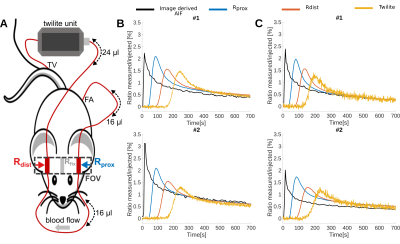
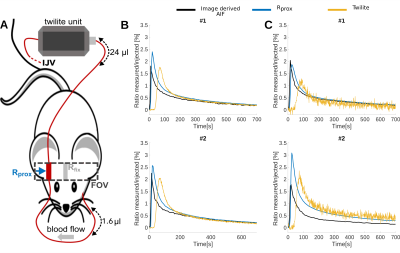
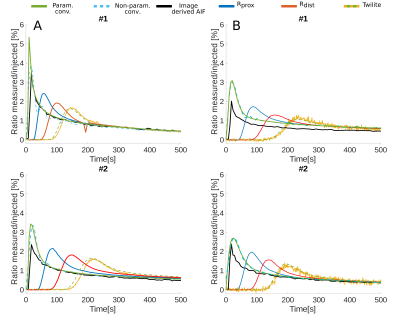
Overall 23 female NMR nu-/nu- mice were measured in a 9.4 T Bruker BioSpec small animal MRI. All mice had intracranial U87 glioblastoma tumors. Either 100 µl of 35 mM Gd-DO3A-butrol (Gadovist) or 100 µl Gd-DTPA (Magnevist) were mixed with their radioactive analogs [68Ga]-Ga-DO3A-butrol (5.74 ± 2.96 Mbq) or [99m Tc]-Tc-DTPA (23.78 ± 4.08 Mbq) and simultaneously injected. An extracorporeal circulation was applied either from the femoral artery to the tail vein (n = 13) or from the carotid bifurcation to the jugular vein (n = 10). The circulations featured MR measurement reservoirs with a volumetric distance of 16.1 µl from the femoral artery and a negligible distance from the carotid artery (1.6 µl). Further downstream, blood radioactivity was assessed in a MR compatible measuring unit (Swisstrace, Twilite) and additionaly, laser doppler flow measurements were performed. A Golden-angle Radial Sparse Parallel (GRASP) sequence (approximately 0.195 x 0.195 x 0.195 mm3, 5 s/frame, TR/TE 4/0.011 ms, FA 13.4°)2, combined with a compressed sensing MP2RAGE sequence for T1 mapping (approximately 0.195 x 0.195 x 0.195 mm3, TI1/TI2/MP2RAGETR = 800 ms/2200 ms/6250 ms, TE/TR = 3.253/7 ms, FA 7°)3, was used for DCE-MRI (Figure 1). Contrast agent concentrations were calculated as previously described 1 and were based on Relaxation Rates of r1 = 4.1 mM−1s−1 for Gadovist and r1 = 2.94 mM−1s−1 for Magnevist. T1 values were individually measured with cs-MP2RAGE for extracorporeal blood. Literature value of T1 = 2400 ms was taken for intracorporeal blood. Radioactive tracer concentrations were determined based on a separately measured sensitivity factor. Deconvolution of the resulting AIFs from the proximal reservoir were computed by performing two different approaches, either a parametric model using a fitting routine a non-parametric model, using a regularized iterative least square method. Both were depending on the estimation of the dispersion kernel, derived by the convolution between the AIF extracted from the distal reservoir and a single gamma variate.Results:
Vascular access was experimentally feasible for the femoral artery and vessels of the neck. Whereas dispersion correction was necessary for the femoral artery setup, curves were not significantly dispersed for the carotid artery setup, where curve geometry closely resembled intracorporeal measurements form retroocular vessels (Figures 2-4). Both access ways showed a high degree of correspondence between extracorporeally determined contrast agent concentrations and simultaneously acquired recordings from radiotracer analogs (Figure 2-4) for both contrast agent / radiotracer analog pairs. Similarly for 10 mice measured with the femoral artery setup, equal good correspondence from blood samples, between MRI and ICP-MS determined Gadolinium concentrations was observed (mean deviation 8.7 ± 28 %). In contrast, correspondence of intracorporeally derived AIFs from retroocular vessels was highly variable and overall inferior to extracorporeal measurements. Dispersion effects in the femoral artery shunt were successfully corrected using kernels based on single gamma variate functions, resulting in AIFs resembling the carotid shunt where dispersion seemed negligible (Figure 3-4). Both dispersion correction strategies demonstrated exceedingly realistic AIFs with kernels resulting in excellent fits e.g., R2 > 0.9997.Discussion
Shunt flows varied across different animals but were rather constant in individual measurements. The resulting impact of varying shunt flow on deconvolution strategies isn´t fully solved yet. Therefor it has to be determined in the future, unclear which vascular access way is more robust and precise in subsequent PKM.Conclusion
Independent of the vascular access way, extracorporeal derived AIFs seem to be a promising and feasible strategy for determination of AIFs in mice with high quantitative precision. Consistency of the two dispersion models in individual mice prove the use of gamma variate functions as dispersion kernels. The use of novel rapid imaging techniques in combination with extracorporeal AIF bears a high potential to set the basis for precise quantitative dual PET/MRI PKM in mice.Acknowledgements
The project was supported by the SFB CRC 1450 and the DFG-NAMT application 446292030 of Dr. med. Philipp Backhaus. The project critically relied on support of technical staff, especially Roman Priebe, Dirk Reinhardt, Nina Kreienkamp and Irmgard Hoppe.References
1. Backhaus P, Büther F, Wachsmuth L, et al. 2020, 'Toward precise arterial input functions derived from DCE-MRI through a novel extracorporeal circulation approach in mice', Magn Reson Med, July 2019, 1-12
2. Zhang J, Feng L, Otazo R, Kim SG. 2018, 'Rapid dynamic contrast-enhanced MRI for small animals at 7T using 3D ultra-short echo time and golden-angle radial sparse parallel MRI', Magn Reson Med, April 2018, 140-152
3. Trotier AJ, Rapacchi S, Faller TL, Miraux S, Ribot EJ, 'Compressed-Sensing MP2RAGE sequence: Application to the detection of brain metastases in mice at 7T', Magn Reson Med, June 2019, 551-559
Figures
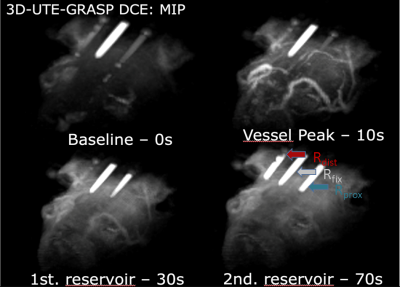
Figure 1: 3D maximum intensity projection (MIP) rendering of DCE-MRI dynamics (reconstructed with T = 5 seconds and λ = 1%) at different stages with femoral artery setup: 1st baseline (0 seconds), 2nd vessel peak (10 seconds), 3rd signal arrival in proximal (Rprox) reservoir (30 seconds), 4th signal arrival in distal (Rdist) reservoir (70 seconds). A reservoir (Rfix) with a fixed amount of contrast agent (0.5 mM) was placed in the middle of the two reservoirs for subsequent drift correction.