0860
Prospective 3D FatNav motion correction for 7T Terra1Clinical Neurosciences, Wolfson Brain Imaging Centre, Cambridge, United Kingdom, 2Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Ultra-high field (7T) imaging can deliver images of brain structure and function at previously inaccessible spatial resolutions down to ~100um. However, subject motion, particularly for patients causes blurring which can lose some of the benefit of 7T imaging. The 3D FatNav approach to motion correction embeds short fat-excitation imaging modules in dead-time in an MRI pulse sequence. We are developing a prospective FatNav module for Siemens 7T Terra scanners. We present here a prospective FatNav module that embeds FatNav into a host pulse sequence, that separates FatNav and main image data during online reconstruction, that computes motion updates and that applies these in GRE imaging sequence in real time.
Introduction
Motion artefacts have been a problem since the very first MRI scans. Protocols with longer acquisition times and studies in patients can both lead to significant motion artefacts. Many approaches have been suggested to mitigate motion effects in MRI but there is not yet a universally accepted solution.1-4 The problem of motion is even more critical at ultra-high field (7T), where images can be acquired down to 100µm spatial resolution. Since even normal breathing causes ~1mm brain motion and the beating heart produces ~100µm brain displacements, motion affects 7T MRI studies even with compliant subjects.5The “FatNav” approach of embedding 3D fat-excitation navigator images into an MR pulse sequence has shown promise for motion correction in the brain.6 So far, FatNav has mainly been implemented retrospectively – i.e. the acquired imaging coordinates are not updated during the acquisition, but instead images are re-gridded post-hoc. However, we believe that a prospective implementation would be beneficial to release the full clinical potential of FatNav. To our knowledge, prospective FatNav is not yet available on the Siemens platform. Our goal is to implement FatNav prospectively for 7T Terra scanners.
Methods
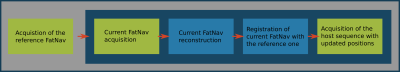
Figure 1. shows a schematic of the general pipeline for the prospective FatNav, which starts with a highly accelerated fat-excitation imaging module, followed by online reconstruction of this low resolution FatNav image and co-registration with the first (reference) FatNav image. The extracted transformation matrix is sent to the host pulse sequence, which updates its slice coordinates in the magnet-fixed coordinate frame before the next host image volume is acquired. This scheme is repeated throughout the host image acquisition.We modified the product GRE sequence by inserting a custom module with fat excitation and accelerated 3D acquisition to acquire fat navigator images. We also added realtime feedback to update the imaging coordinate system.
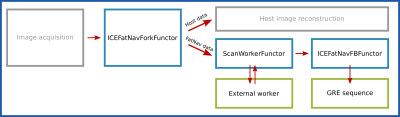
We modified the standard ICE reconstruction pipeline as shown in Figure 2.
We added a functor “ICEFatNavForkFunctor” to separate the FatNav data out of the main reconstruction chain. The FatNav data are then sent to “ScanWorkerFunctor” which launches an external worker process and transfers FatNav raw data to it and receives motion updates in return. The worker process accumulates FatNav raw data, and whenever a complete single dataset is ready it calls a Matlab script to reconstruct the 3D FatNav image. The worker process then calls FSL FLIRT running in a chroot on the MARS to coregister the FatNav image against the first (reference) FatNav image and return the transformation matrix. This is sent back to the host sequence by the “ICEFatNavFBFunctor”.
For acquisition of the navigators a 3D fat excitation block with the parameters matching previous retrospective studies6 was prepared, i.e. Matrix = 128x128x44, FOV = 256x256x88mm GRAPPA = 4x4, Partial Fourier = 6/8 (in both in-plane and through-plane directions), TE = 1.49ms, TR = 3.2ms, FA = 7°, 3s total time.
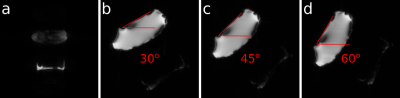
In order to validate sequence motion updates, we acquired several scans of an immobile phantom, while sending known transformation matrices to the sequence (Fig. 3.). The phantom consisted of fat compartment (margarine box) at the bottom and a plastic bag with aqueous glucose solution above.
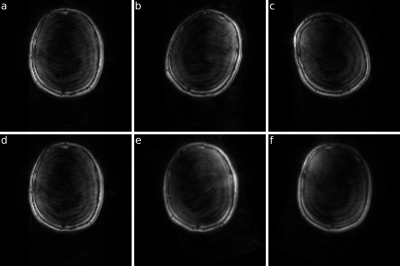
As a proof of concept, we scanned a healthy volunteer who was asked to rotate his head at defined moments while scanning (Fig. 4.). The host 2D GRE sequence was following: 128x128 matrix, 260x260mm2 matrix (150x150mm2 for phantom study), 10ms TE, 100ms TR, 25° FA.
Results
Sequence motion updates were tested on the phantom and showed that the host sequence properly corrects shifts and rotations in each direction.In vivo scan of the healthy volunteer confirmed that each module of the presented prospective FatNav pipeline works properly giving as a result prospectively corrected for motion host images.
Discussion
Presented results create basis for implementation for prospective FatNav motion correction. Its modular structure should guarantee relative ease of implementation to other sequences and further optimisation of particular modules. We now aim to improve the feedback time sufficiently for clinical studies on patients with movement control disorders.Conclusion
We implemented a modular framework for FatNav motion correction on the Siemens 7T Terra platform We need to reduce the feedback time before using this in clinical studies.Acknowledgements
CTR is funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society [098436/Z/12/B].
This study was funded by the NIHR Cambridge Biomedical Research Centre and MRC Clinical Research Infrastructure Award for 7T.
References
1. J. G. Pipe, Motion Correction With PROPELLER MRI: Application to Head Motion and Free-Breathing Cardiac Imaging. MRM. 1999;42:963-969.
2. J.M. Pauly, S.M. Conolly, D.G. Nishimura, et al., Slice-selective excitation for very short T2 species, in: Proc. 8th Annu. Meet. SMRM, Amsterdam, 1989:28.
3. M. Weiger, K. P. Pruessmann, MRI with Zero Echo Time. EmagRes. 2012;2(1):311-321.
4. P. Huang, J.D. Carlin, A. Alink, et al., Prospective Motion Correction improves the sensitivity of fMRI pattern decoding. Hum Brain Mapp. 2018;39(10):4018-4031.
5. J. Maclaren, B.S. Armstrong, R.T. Barrows, Measurement and correction of microscopic head motion during magnetic resonance imaging of the brain. PloS One 7. 2012 e48088.
6. D. Gallichan, J. P. Marques, R. Gruetter, Retrospective Correction of Involuntary Microscopic Head Movement Using Highly Accelerated Fat Image Navigators (3D FatNavs) at 7T.
Figures