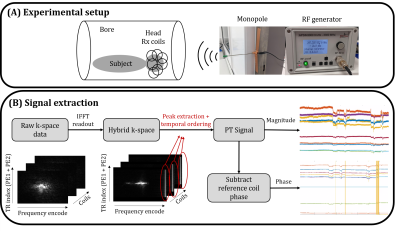
0859
Pilot Tone meets DISORDER: Improved data-driven motion-corrected brain MRI by leveraging Pilot Tone signal variations1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3London Collaborative Ultra high field System (LoCUS), London, United Kingdom, 4Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid and CIBER-BNN, Madrid, Spain, 5MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
DISORDER is an established retrospective data driven motion correction approach that uses optimised phase encoding, but otherwise unmodified 3D acquisitions. It is highly effective, but requires multiple lines of k-space to be grouped together for each motion state to be estimated, and this limits temporal resolution. At 7T, head motion can also be detected by “Pilot Tone”, which is an injected RF signal picked up by each coil in the head receiver array, but a calibration step is required. Here we combine DISORDER and Pilot Tone to achieve integrated calibration and show that improved motion correction can result.
Introduction
Brain MRI is susceptible to motion which causes artefacts in reconstructed images1, especially with prolonged scanning times for high-resolution MRI, particularly relevant at ultra-high field (UHF)2. Data-driven motion correction proposed in DISORDER3,4 leverages an optimised Cartesian sampling to retrospectively correct for rigid body motion during volumetric acquisitions. Whilst able to correct UHF acquisitions5, stable motion estimates require multiple readout lines to be assigned to a single motion state, limiting the temporal resolution of estimates. In this work, we extend DISORDER motion estimation by incorporating external motion information coming from injected Pilot Tone (PT) signals6 potentially improving the temporal resolution of motion estimates.Methods
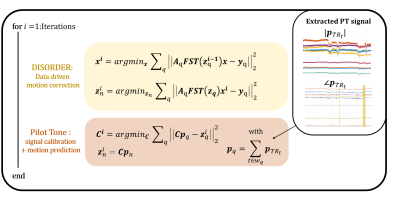
DISORDER motion correction3,4 divides the k-space acquisition into temporal groups (sweeps) of readouts (one per repetition time TR) that sample uniformly across k-space. Each sweep $$$n$$$ is treated as a different motion state with rigid motion parameters $$$\textbf{z}_n$$$ that can be jointly optimised together with the image $$$\textbf{x}$$$:$$(\widehat{\textbf{x}},\widehat{\textbf{z}_n})=argmin_{\textbf{x},\textbf{z}_n}\sum_q\Big\| \textbf{A}_q\textbf{FST}(\textbf{z}_q)\textbf{x}-\textbf{y}_q \Big\|_2^2 \quad\quad(1)$$
where $$$q$$$ is the running variable summing over all sweeps with $$$\textbf{T}(\textbf{z}_q)$$$,$$$\textbf{S}$$$,$$$\textbf{F}$$$,$$$\textbf{A}_q$$$ and $$$\textbf{y}_q$$$ respectively representing associated rigid motion, coil sensitivities, Fourier operator, sampling structure, and measured k-space data. PT is a motion-estimation method leveraging signals collected during data acquisition by each receiver channel from an externally generated mono-frequency RF source: PT signals vary slightly with head position and provide updated motion information with every TR6,7. For brain MRI at 7T, it was shown that a linear model can be used to predict motion parameters ($$$\textbf{z}_{{TR}_t}$$$) from the PT signal ($$$\textbf{p}_{{TR}_t}$$$)8:
$$\textbf{z}_{{TR}_t}=\textbf{C}\textbf{p}_{{TR}_t}\quad \quad(2)$$
where $$$\textbf{p}_{{TR}_t}$$$ is a 33-element complex vector containing 32-channel PT signal for the TR at time $$$t$$$ and a 1 to allow for motion offsets. $$$\textbf{C}$$$ is a 6x33 matrix with coefficients for each of the 6 rigid motion parameters in each row. This allows estimating $$$\textbf{C}$$$:
$$(\widehat{\textbf{x}},\widehat{\textbf{C}})=argmin_{\textbf{x},\textbf{C}}\sum_q\Big\|\textbf{A}_q\textbf{FST}(\textbf{C}\textbf{p}_{q})\textbf{x}-\textbf{y}_q \Big\|_2^2 \quad \quad(3)$$
where $$$\textbf{p}_{q}$$$ is the averaged PT signal within the time window $$$w_q$$$ of shot $$$q$$$. Whereas in Equation 1 each motion state is estimated by optimising for an independent quantity, the matrix $$$\textbf{C}$$$ is assumed to be constant for the full acquisition, which can stabilise motion states over time. Implementation details for solving Equation 3 are shown in Figure 2.
The proposed reconstruction was tested in simulations and validated on 4 healthy volunteers (HV) (aged 25-35yrs, Institutional Research Ethics number HR-18/19-8700) with a mix of deliberate motions and lying still, using a volumetric SPGR acquisition on a 7T scanner (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany). Sequence parameters: $$$0.5^3mm^3$$$ isotropic resolution, DISORDER random checkered sampling with $$$4.1s$$$ per sweep, $$$TR=8.4ms$$$, echo time $$$TE=4.2ms$$$, flip angle $$$FA=7^{\circ}$$$, field of view (FOV)=248x238x176 (IS/AP/RL), readout along IS, scan duration $$$TA=19min49s$$$ with no repeats and no acceleration. The PT was generated with an RF signal generator (APSIN3000, AnaPico, Glattbrugg, Switzerland) and broadcast into the magnet room from a monopole antenna at a drive level of $$$-20dBm$$$ (Figure 1a) and with an offset to set the signal in the oversampled FOV, which was extracted before removing the oversampling (Figure 1b). Images are first reconstructed using motion parameters obtained from solving Equation 1 and $$$\textbf{C}$$$ was then calibrated using Equation 2 to obtain the final reconstructions from the PT consistent motion parameters. Due to computational constraints, full volumetric data was reconstructed at $$$1mm^3$$$ and the motion information (either temporal motion estimates or the calibrated matrix ) was then used to reconstruct single slabs at $$$0.5^3mm^3$$$.
Results
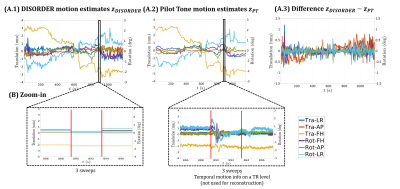
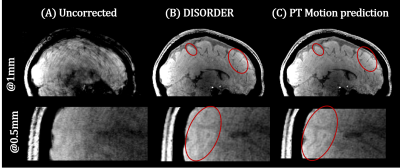
Figure 3a shows the estimated motion parameters with DISORDER compared to PT predicted motion states. These agree well overall, with cleaner estimates using the PT calibration. Zoomed-in traces (Figure 3b) show the higher temporal resolution motion estimates PT provides (per TR). Example reconstructed images in Figure 4 show successful motion correction using DISORDER, but further improvements using the PT predicted motion information. PT reconstructions for all subjects showed either similar or improved image quality compared to DISORDER.Discussion
We have investigated and integrated PT signals to improve DISORDER motion correction. PT signal calibration can be achieved using the DISORDER motion states and the two then show a strong correlation, but the PT estimates are more stable and have higher temporal resolution. Replacing DISORDER motion estimates with PT either produced similar results or improved motion correction. As shown in Figure 3, PT can provide per TR motion estimates, which look highly coherent. It seems plausible that motion correction at the TR level could yield further improvements but this has hitherto proved computationally infeasible with the current framework.Conclusion
We have exploited the use of PT at UHF to improve data-driven motion correction. Our method does not interfere with sequence parameters and can be used in almost any sequence. PT predicted motion states can improve image quality and offer new ways to retrospectively detect and correct for motion at a higher temporal resolution, potentially with individual motion states for each k-space line (i.e. at the TR level).Acknowledgements
The authors would like to thank Francesco Padormo for the loan of the RF signal generator.
This work was funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging [EP/L015226/1] and supported by a Wellcome Trust Collaboration in Science Award [WT 201526/Z/16/Z] and the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z].
This work was supported by Wellcome Trust Collaboration in Science grant [WT201526/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
[1] Zaitsev, Maxim, Julian R. Maclaren and Michael Herbst. “Motion artifacts in MRI: A complex problem with many partial solutions.” Journal of Magnetic Resonance Imaging 42 (2015): 42(4):887-901.
[2] Stucht, Daniel, Kadashevich Appu Danishad, Peter Schulze, Frank Godenschweger, Maxim Zaitsev and Oliver Speck. “Highest Resolution In Vivo Human Brain MRI Using Prospective Motion Correction.” PLoS ONE 10 (2015).
[3] Cordero-Grande, Lucilio, Giulio Ferrazzi, Rui Pedro A. G. Teixeira, Jonathan O’Muircheartaigh, Anthony N. Price and Joseph V. Hajnal. “Motion‐corrected MRI with DISORDER: Distributed and incoherent sample orders for reconstruction deblurring using encoding redundancy.” Magnetic Resonance in Medicine 84 (2020): 713 - 726. [4] Cordero-Grande, Lucilio, Rui Pedro A. G. Teixeira, Emer J. Hughes, Jana Hutter, Anthony N. Price and Joseph V. Hajnal. “Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction.” IEEE Transactions on Computational Imaging 2 (2016): 266-280.
[5] Cordero-Grande L, Tomi-Tricot R, Ferrazzi F, Sedlacik J, Malik SJ, Hajnal JV. Preserved high resolution brain MRI by data-driven DISORDER motioncorrection. In: Proc Int Soc Mag Reson Med 20.
[6] Bacher M. Cardiac Triggering Based on Locally Generated Pilot-Tones in a Commercial MRI Scanner: A Feasibility Study. October 2017.
[7] Vahle, Thomas, Mario Bacher, David Rigie, Matthias Fenchel, Peter Speier, Jan Bollenbeck, Klaus P. Schäfers, Berthold Kiefer and Fernando E. Boada. “Respiratory Motion Detection and Correction for MR Using the Pilot Tone: Applications for MR and Simultaneous PET/MR Examinations.” Investigative Radiology (2019).
[8] Wilkinson T, Godinez F, Brackenier Y, Tomi-Tricot R, Cordero-Grande L Bridgen P, Giles S, Hajnal JV, Malik SJ. Motion Estimation for Brain Imaging at Ultra-High Field Using Pilot-Tone: Comparison with DISORDER Motion Compensation. In: Proc Int Soc Mag Reson Med 21.
Figures