0858
Motion-corrected supine breast MRI with online reconstruction1IADI, Université de Lorraine, INSERM, Nancy, France, 2High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 3CIC-IT, CHRU de Nancy, INSERM, Nancy, France
Synopsis
A standard breast MRI protocol implies prone position. Supine position has multiple advantages; however, it requires motion correction. In this work we present our results on retrospective nonrigid motion correction of supine breast MRI on 7 healthy volunteers. A respiratory belt was used to capture the chest movements. Images were reconstructed online and fully automatically, including physiological data recording and its transfer to the reconstruction server. It was shown that application of the selected motion correction method improves image sharpness on 32-47% which was cofirmed by visual observations.
Introduction
MRI is the most preferable modality of breast cancer screening for younger women who have denser breast tissues [1]. A standard breast MRI protocol implies utilization of a dedicated coil. Women should lie in the prone position so that the breast tissues spread freely into the coil recesses. The prone position prevents the respiratory motion artifacts; however, staying still in the prone position is extremely uncomfortable and complicates mapping of radiological findings on actual operational or biopsy position, which is usually supine. Different strategies were applied to achieve artifact-free breast MRI in supine position. Expiratory gating helps to avoid the respiratory motion artifacts [2], however the acquisition time greatly increases in this case. A combination of phase-encode reordering and gating for motion compensation reduces motion artifacts sufficiently to ensure a diagnostic quality [3]. In this work we present our results on motion-corrected breast MRI in supine position with the reconstruction done online.Methods
The participants were 7 healthy women of age 23-49 years and provided a written consent. The acquisition was performed on a Siemens Prisma 3T (Siemens, Erlangen, Germany). The protocol included T2-weighted TSE (turbo spin echo) sequence with 60 axial slices acquired in two different patient positions: prone with a conventional breast coil and supine with a cardiac coil. In-plane resolution was 0.72 mm and slice thickness was 3 mm. 3 participants of 7 (participants 3-5) wore a flexible breast holder in order to decrease gravitational breast deformation. A respiratory belt (Maglife, Schiller Médical, Wissembourg, France) was put on the patients’ chest to measure their respiratory activity in supine position. The respiratory belt indications were automatically recorded by the signal analyzer and event controller system [4] and sent on the reconstruction server.The image reconstruction in supine position was performed online using Gadgetron framework [5] and compared with the manufacturer’s reconstruction. Motion-corrected reconstruction was done with the generalized reconstruction by inversion of coupled systems (GRICS) method [6]. This algorithm solves for both a motion-corrected image ρ0 and the parameters of a motion model α, that links respiratory belt indications with actual nonrigid in-plane motion, in two coupled steps called Generalized reconstruction and Motion model optimization, respectively:
$$\begin{cases}s=E(\alpha)\rho_0, \\ \varepsilon(\rho_0,\alpha,\delta\alpha) = R(\rho_0,\alpha)\delta\alpha.\end{cases}$$
Here $$$s$$$ is the acquired MRI signal, $$$E$$$ is an encoding operator and linear operator $$$R$$$ links modification of the motion model $$$\delta\alpha$$$ and the reconstruction residue $$$\varepsilon$$$. The optimization is done alternating these two steps on different scales staring from the lowest resolution where all movement can be considered as small, iteratively going to the image native resolution. The images were normalized by pixel-wise division by sum of squares of the channels. Reconstruction in prone position was done with the manufacturer’s algorithm. Image quality of each image was estimated using a sharpness index SI [7]. Sharpness enhancement was calculated as $$$SE = (SI_{GRICS}-SI_{std})/(SI_{GRICS}+SI_{std})$$$, where $$$SI_{GRICS}$$$ is the sharpness index of motion-corrected images and $$$SI_{std}$$$ is the sharpness index of the images reconstructed with the standard manufacturer’s algorithm. In order to exclude the impact of residual motion and aliasing artifacts which manifest more outside of the breast, i.e. outside of the region of interest, the images were automatically segmented with a k-means based segmentation algorithm and the background was masked out.
Results
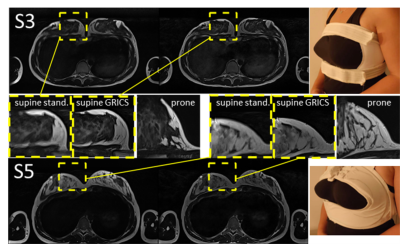
Examples of images reconstructed with both GRICS and the standard algorithm are shown in Figure 1. Zoomed regions aim to demonstrate the fine features of the milk glands, and the regions of images acquired in prone position allow visual comparison of the motion-corrected and similar quasi-motionless images. It can be seen that the motion blurring present on the standard images, is manifestly reduced on the motion-corrected images making their quality comparable with that of the images acquired in prone position.Sharpness indices and sharpness enhancements calculated from the masked images were as in the Table 1.
Discussion
It can be seen that utilization of the breast holders does not seem to decrease the image quality, while helping to reduce impact of gravitation on the breast shape.Despite image quality is comparable with that of prone MRI images, the signal-to-noise ratio could still be improved. A flexible coil (“BraCoil”) could be a solution, however its utilization complicates correct installation of the respiratory belt. Preliminary tests have shown that MARMOT sensor [8] gives promising improvement on image quality.
Conclusions
We have shown success of the proposed motion correction algorithm based on respiratory belt indications in case of breast MRI in supine position. Resulting images quality is comparable with that of the prone breast MRI. Sharpness index calculations show its dramatic increase for the motion-corrected images. Future steps will include utilization of a flexible coil and an accelerometer instead of a pneumatic respiratory belt.Acknowledgements
This work was supported by “BRACOIL“ project (Agence Nationale de Recherche, France ANR-17-CE19-0022/Austrian Science Fund FWF Nr. I-3618).References
1. Bruening, W et.al. (2012). Noninvasive diagnostic tests for breast abnormalities: update of a 2006 review.
2. Janssen, N. N., et.al.. (2017). Supine breast MRI using respiratory triggering. Academic radiology, 24(7), 818-825.
3. Siegler, P., et.al. (2011). Supine breast MRI. Journal of Magnetic Resonance Imaging, 34(5), 1212-1217.
4. Odille, F., et.al. (2007). Noise cancellation signal processing method and computer system for improved real-time electrocardiogram artifact correction during MRI data acquisition. IEEE Transactions on Biomedical Engineering, 54(4), 630-640.
5. Hansen, M. S., & Sørensen, T. S. (2013). Gadgetron: an open source framework for medical image reconstruction. Magnetic resonance in medicine, 69(6), 1768-1776.
6. Odille, F., Vuissoz, P. A., Marie, P. Y., & Felblinger, J. (2008). Generalized reconstruction by inversion of coupled systems (GRICS) applied to free‐breathing MRI. Magnetic Resonance in Medicine, 60(1), 146-157.
7. Leclaire, A., & Moisan, L. (2015). No-reference image quality assessment and blind deblurring with sharpness metrics exploiting fourier phase information. Journal of Mathematical Imaging and Vision, 52(1), 145-172.
8. Chen, B., Weber, N., Odille, F., Large-Dessale, C., Delmas, A., Bonnemains, L., & Felblinger, J. (2016). Design and validation of a novel MR-compatible sensor for respiratory motion modeling and correction. IEEE Transactions on Biomedical Engineering, 64(1), 123-133.
Figures