0857
Prospective Motion Correction in Kidney MRI Using Free Induction Decay Navigators1Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 2Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 3Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Abdominal MRI scans may require breath-holding to prevent image quality degradation, which can be challenging for patients. In this study, we used free induction decay navigators (FIDnavs) to correct motion prospectively. A short calibration scan was acquired prior to the prospectively corrected scan to create a linear motion model to translate the FIDnav signal into kidney displacement. Shallow, deep and continuous deep breathing scans were acquired with the proposed technique and reconstructed images were compared to those without motion correction. The proposed technique reduces blurriness and motion artifacts in the kidneys by correcting their position prospectively during the MRI acquisition.
Introduction
Abdominal MRI scans are used for the clinical assessment of several diseases such as hydronephrosis and Crohn's disease. These scans may require breath-holding to achieve sufficient image quality which might be challenging for patients, especially for children1. Free-breathing imaging with radial acquisition is relatively robust to motion; however, image quality is often degraded when imaging nervous and sick patients who are breathing deeply. Most motion correction techniques for abdominal MRI are based on retrospective correction which bins data according to respiratory phases assuming that the subject is breathing regularly2 or discards the motion corrupted data3. Detected motion may also be used for prospectively gating; however, this reduces the efficiency of the acquisition4. We propose to use free induction decay navigators (FIDnavs)5,6 to measure motion and apply motion correction (MoCo) prospectively with real-time steering by estimating kidney displacement due to respiration during the acquisition in abdominal MRI.Methods
FIDnavs with their own non-selective RF pulses were inserted into a golden angle-ordered radial stack-of-stars sequence. FIDnavs (ADC duration=0.4ms, TE=2 ms) were acquired once in each partition (or equivalently once in each rotation angle), hence acquired once in every TR x Number of Slices. Six volunteers (3 female, 3 male, age=37.4±10.7 years) were scanned at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) following written, informed consent. Supine and body matrix coils were used. For each volunteer, a short calibration scan was acquired, before the real-time motion correction scan to estimate the linear model parameters. Estimated model parameters were fed into deep breathing and continuous deep breathing scans with MoCo.Calibration scan: Imaging parameters were TE/TR/FA = 1.49ms/4ms/9˚, 16 coronal oblique slices with slice Partial Fourier=6/8, voxel size=2.4x2.4x6mm3, 900 radial spokes, resulting in a total scan time of 50 seconds. In the calibration scan, FIDnavs are acquired in every ~50 ms. The tilt angle of the imaging plane was selected to align with the angle of the kidneys. In the calibration scan, volunteers were asked to perform inhaling/exhaling alternatively every 5 seconds in total for 50 seconds. The calibration scan was reconstructed using inverse non-uniform fast Fourier transform (iNUFFT)7. The position of the kidneys in oblique yz-direction (anterior-posterior & head-foot) were manually labeled on reconstructed volumes with a temporal resolution of 5 seconds, corresponding to each exhale and inhale state (Figure 1). The correlation coefficients between kidney displacement and the magnitude of the FIDnav measured by each coil (FIDnavcoilk) were computed and the coil with the highest correlation coefficient was determined (coilmax). A linear motion model was used to estimate translation in oblique yz-direction:
$$$\Delta yz_{kidney}(t) = \boldsymbol{a}\:x\:(FIDnav_{coilmax}(t)-FIDnav_{coilk}(0)) \;[Eqn. 1]$$$.
The value for a was calculated and used for real-time motion correction.
Real-time motion correction experiments: The coil identity number and calculated linear motion model parameter (a) were fed into the sequence. During the acquisition, MoCo was applied prospectively based on the Kalman-filtered estimated kidney displacement using Eqn. 1. For deep breathing (DB), volunteers performed exhaling and inhaling with 10 seconds duration for each state. For continuous deep breathing (CDB), volunteers continuously exhaled and inhaled for 5 seconds alternatively. The total scan time was 2.5 minutes. Imaging parameters were TE/TR/FA=1.49/4ms/9˚, 32 coronal oblique slices with slice Partial Fourier=6/8, voxel size=1.2x1.2x3mm3 and 1326 radial spokes. In real-time MoCo scans, FIDnavs were acquired and MoCo parameters were updated every ~100 ms. DB and CDB scans were repeated without applying MoCo. In addition, volunteers were scanned without MoCo while they performed shallow breathing (SB). Using GRASP reconstruction8, dynamic volumes for inhale/exhale states were obtained. Dice scores between reconstructed inhale/exhale frames were computed to evaluate the efficacy of the correction. Single volumes were also reconstructed using all of the acquired spokes to assess image quality with and without MoCo.
Results
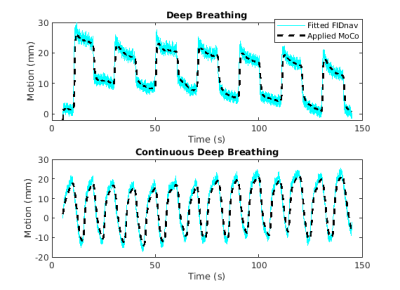
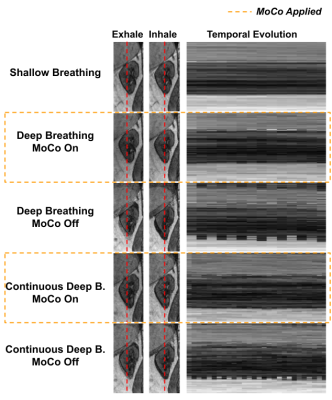
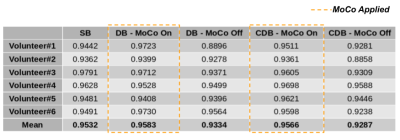
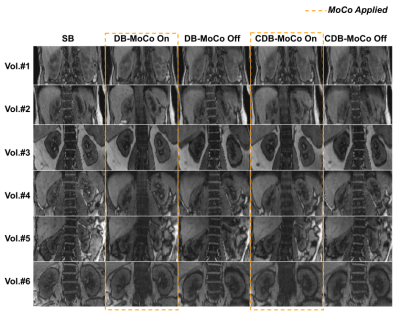
Examples of fitted FIDnavcoilmax and Kalman-filtered FIDnavcoilmax (or equivalently applied MoCo) for deep breathing and continuous deep breathing are depicted in Figure 2. GRASP reconstructions for inhale-exhale states and time evolution of reconstructed dynamic volumes for SB, DB, and CDB with MoCo and without MoCo scans are shown in Figure 3 for a representative volunteer. Table 1 depicts the computed Dice score values between inhale/exhale frames. Dice scores were improved from 0.93 to 0.96 using MoCo for both deep breathing and continuous deep breathing experiments. Single volume reconstructions for all volunteers are displayed in Figure 4.Discussion and Conclusion
Temporal evolution of the reconstructed dynamic image series demonstrates that scans with MoCo have better-aligned series of volumes over time (Figure 3). In Table 1, it is observed that Dice scores computed between inhale/exhale frames are improved by MoCo. The proposed MoCo technique reduces blurriness and motion artifacts in kidneys by correcting their position (Figure 4) and is expected to result in a more reliable clinical assessment.For the calibration scan, we acquired in total 10 inhale/exhale states in 50 seconds. However, in the future, this can be shortened by acquiring fewer inhale/exhale states. In the proposed work, the motion of the kidneys due to respiration was assumed as rigid motion, hence artifacts due to non-rigid motion may remain, which can be corrected retrospectively for improved image quality. The same pipeline can be applied to other organs such as the liver.
Acknowledgements
This work was supported in part by the grant No 2019056 from the United States-Israel Binational Science Foundation (BSF), the Boston Children's Hospital Translational Research Program Pilot Grant 2018, Society of Pediatric Radiology Multi-center Research Grant 2019, Crohn’s and Colitis Foundation of America’s (CCFA) Career Development Award and the National Institutes of Health under award numbers R21DK123569, S10OD0250111, R01EB019483, R21EB029627.References
1. Chavhan, Govind B., Paul S. Babyn, and Shreyas S. Vasanawala. "Abdominal MR imaging in children: motion compensation, sequence optimization, and protocol organization." Radiographics 33.3 (2013): 703-719.
2. Feng, Li, et al. "XD‐GRASP: golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing." Magnetic resonance in medicine 75.2 (2016): 775-788.
3. Coll Font, Jaume, et al. "Bulk motion‐compensated DCE‐MRI for functional imaging of kidneys in newborns." Journal of Magnetic Resonance Imaging 52.1 (2020): 207-216.
4. Brau, Anja CS, and Jean H. Brittain. "Generalized self-navigated motion detection technique: preliminary investigation in abdominal imaging." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 55.2 (2006): 263-270.
5. Kober, Tobias, et al. "Head motion detection using FID navigators." Magnetic resonance in medicine 66.1 (2011): 135-143.5.
6. Wallace, Tess E., et al. "Head motion measurement and correction using FID navigators." Magnetic resonance in medicine 81.1 (2019): 258-274.6.
7. Fessler, J.A. and Sutton, B.P., 2003. Nonuniform fast Fourier transforms using min-max interpolation. IEEE transactions on signal processing, 51(2), pp.560-574.
8. Feng, Li, et al. "Golden angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden‐angle radial sampling for fast and flexible dynamic volumetric MRI." Magnetic resonance in medicine 72.3 (2014): 707-717.
Figures