0833
The lipid signal in cervical cancer: a 1H MRS pilot study1Core Facilities, Istituto Superiore di Sanita', Rome, Italy, 2Department of Radiological, Oncological and Pathological Sciences, Policlinico Umberto I Sapienza University of Rome, Rome, Italy, 3Department of Physics and Astronomy, University of Bologna, Bologna, Italy, Rome, Italy
Synopsis
Cervical cancer (CC) is the fourth most common malignancy and cancer-related mortality cause in women worldwide. A significant portion of these patients are diagnosed as locally advanced cervical cancer (LACC), thus requiring combined therapies. We investigate the role of the lipid signal derived from 1H magnetic resonance (MR)-spectroscopy in assessing response to neoadjuvant chemotherapy of LACC.
Introduction
Cervical cancer (CC) is the fourth most common malignancy and cancer-related mortality cause in women worldwide, with a higher prevalence in developing countries.1 Surgery represents the gold standard in early-stage CC. However, a significant portion of these patients are already diagnosed as locally advanced cervical cancer, thus requiring combined therapies, such as concurrent chemo-radiotherapy (CCRT) or neoadjuvant chemotherapy (NACT) combined with radical surgery (RS).2 Due to its excellent soft-tissue characterization, MRI represents the imaging technique of choice for the staging of CC, playing a fundamental role in assessing therapeutic strategy and response to therapy3. Besides the anatomical characterization provided by conventional MRI, localized proton magnetic resonance spectroscopy (1H MRS) allows in vivo metabolic characterization of a suspicious lesion, giving non-invasive information useful for a more accurate and faster diagnosis4. Few studies have applied MRS to distinguish between benign and malignant gynecologic lesions, including CC5-6 or as a response biomarker in cervical cancer7, but with uncertain results.Purpose
The purpose of our study was to investigate the role of the lipid peak derived from 1H magnetic resonance (MR)-spectroscopy in assessing cervical cancer (CC) prognosis, particularly in assessing response to neoadjuvant chemotherapy of locally advanced cervical cancer (LACC).Methods
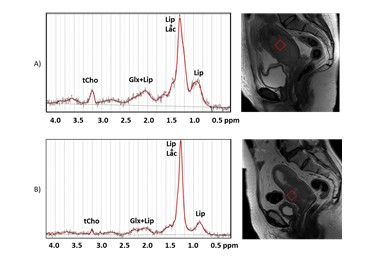
We enrolled 17 patients with histologically proven CC who underwent MR at baseline. Images and spectra were acquired using a superconducting magnet operating at a field strength of 3.0 T (GE Discovery MR 750, GE Healthcare, Milwaukee, WI, USA) with an 32-channel phased-array coil positioned on lower abdomen. In addition to conventional sequences for pelvic assessment, the MR protocol included a single-point voxel resolved spectroscopy (PRESS) sequence, with repetition time (TR) of 1500 ms and echo time (TE) of 28 and 144 ms. Voxels (volume 18x18x18 mm3) were positioned in the central part of the tumor, carefully avoiding contamination from surrounding tissues and areas of necrosis. The short echo time (TE=28 ms) was chosen for metabolite quantification, while the longest TE was chosen for the detection of Lac whose signal can be hidden when a large lipid signal (resonating at 1.28 ppm) is also present. Spectra were analyzed using the LCModel fitting routine, thus extracting multiple metabolites, including lipids. A quantitative protocol, which use the unsuppressed water signal as internal standard was also applied.8 11/17 patients were found to be LACC. These patients were treated with neoadjuvant chemotherapy and reassessed at term with MR at 3 months. Patients were then divided into two groups: good responder (GR; response to therapy >50%) and poor responder/non-responder (PR/NR; response <50% or <20% respectively).Results
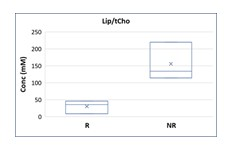
11/17 patients were found to be LACC. Of these 5 patients were excluded from further analysis because 3 were lost to follow-up and 2 had a non-diagnostic spectrum due to a signal-to-noise ratio (SNR) <4. Of the remaining 6 patients, 3 were GR and 3 PR/NR. A statistically significant difference in lipid values was observed in the two groups of patients, in particular with higher Lip values in PR/NR patients than in GR patients, as shown in Figure 1. Furthermore, a significant difference was also observed in the lipid/choline ratio (Lip/tCho), with higher values in PR/NR patients (Figure 2). The absence of lactate in the long echo time spectra (TE=144 ms) excluded lipid overestimation in the quantitative analyses.Conclusions
According to our study, assessment of lipid peak at 1H-MR-spectroscopy could be an additional quantitative parameter in predicting the response to neoadjuvant chemotherapy in patients with LACC.Acknowledgements
No acknowledgement found.References
1) Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191-e203. doi:10.1016/S2214-109X(19)30482-6
2) Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri [published correction appears in Int J Gynaecol Obstet. 2019 Nov;147(2):279-280]. Int J Gynaecol Obstet. 2019;145(1):129-135. doi:10.1002/ijgo.12749
3) Manganaro L, Lakhman Y, Bharwani N, et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018 [published correction appears in Eur Radiol. 2021 Jun 17;:]. Eur Radiol. 2021;31(10):7802-7816. doi:10.1007/s00330-020-07632-9
4) García-Figueiras R, Baleato-González S, Padhani AR, et al. Proton magnetic resonance spectroscopy in oncology: the fingerprints of cancer?. Diagn Interv Radiol. 2016;22(1):75-89. doi:10.5152/dir.2015.15009
5) Booth SJ, Pickles MD, Turnbull LW. In vivo magnetic resonance spectroscopy of gynaecological tumours at 3.0 Tesla. BJOG. 2009;116(2):300-303. doi:10.1111/j.1471-0528.2008.02007.x
6) Mahon MM, Cox IJ, Dina R, et al. (1)H magnetic resonance spectroscopy of preinvasive and invasive cervical cancer: in vivo-ex vivo profiles and effect of tumor load. J Magn Reson Imaging. 2004;19(3):356-364. doi:10.1002/jmri.20012
7) Harry VN. Novel imaging techniques as response biomarkers in cervical cancer. Gynecol Oncol. 2010;116(2):253-261. doi:10.1016/j.ygyno.2009.11.003
8) Canese R, Pisanu ME, Mezzanzanica D, et al. Characterisation of in vivo ovarian cancer models by quantitative 1H magnetic resonance spectroscopy and diffusion-weighted imaging. NMR Biomed. 2012;25(4):632-642. doi:10.1002/nbm.1779