0831
Hyperpolarized dehydroascorbate reveals ascorbate-mediated oxidative stress increases flux through the pentose phosphate pathway in cancer
Nathaniel Kim1, Marjan Berishaj1, Elisa de Stanchina2, Manish Shah3, Lewis Cantley4, and Kayvan Keshari1
1Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Antitumor Assessment Core Facility, Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Weill Cornell Medicine, New York-Presbyterian Hospital, New York, NY, United States, 4Meyer Cancer Center, Department of Medicine, Weill Cornell Medical College, New York, NY, United States
1Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Antitumor Assessment Core Facility, Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Weill Cornell Medicine, New York-Presbyterian Hospital, New York, NY, United States, 4Meyer Cancer Center, Department of Medicine, Weill Cornell Medical College, New York, NY, United States
Synopsis
HP DHA was demonstrated as an imaging agent for probing the biochemical response to ascorbate therapy in patient derived xenograft (PDX) models of cancer. Changes in DHA/ascorbate metabolism were observed in PDX tumors after prolonged treatment, mirroring the observed treatment response to high dose ascorbate. Metabolomic studies showed that high dose ascorbate therapy induced oxidative stress, leading to increased flux through the pentose phosphate pathway (PPP). DNA damage repair mutants exhibit lower baseline flux through the PPP and are more sensitized to ascorbate induced ROS. These results demonstrate a new method for assessing oxidative stress in vivo using HP DHA.
Introduction
Tumor progression and survival is driven by genetic mutations and environmental conditions. Recent work has revealed that redox metabolism is deregulated in pancreatic cancer and may provide new opportunities for diagnosis and targeted treatment. As such, recent studies have sought to understand how changes in metabolic flux in response to oxidative stress can be leveraged for the targeted treatment of cancer. Specifically, a therapeutic strategy for targeting KRAS mutant cancers using high dose ascorbate was introduced to disrupt redox homeostasis.1 Ascorbate can be oxidized in the presence of Reactive Oxygen Species (ROS) to form dehydroascorbate (DHA), which is transported via glucose transporter (GLUT1). After entering the cell, DHA is reduced back to ascorbate, which causes a depletion of the intracellular redox machinery and in turn sensitizes cells to ROS. Each successive turn of this process results in a cyclical increase in oxidative stress in the tumor microenvironment leading to downstream inhibition of DNA damage repair enzymes. Within this therapeutic approach, it is known that enzymes in the DNA damage response pathway can potentially modulate flux by upregulating the pentose phosphate pathway (PPP). While high dose ascorbate is of great interest as an oxidative stress-mediated therapeutic approach for cancer, assessing this therapeutic strategy and its downstream metabolic targets has been challenging. Here, we report hyperpolarized DHA (HP DHA) as a metabolic biomarker for monitoring oxidative stress in vivo, leading to the discovery that high dose ascorbate therapy increases flux through the PPP in cancer.Methods
Ascorbate therapy of pancreatic cancer PDXs – BRAF-driven (PC87), BRCA-driven (HyMad1) and KRAS-driven (PC106) PDX models, created from patient samples and verified using targeted IMPACT sequencing,4,5 were subcutaneously xenografted in the right flank of NSG mice. 2 weeks after implantation, mice were randomly divided into two groups. One group was treated with freshly prepared vitamin C in 400 μL of PBS (4 g/kg) twice a day via IP injection (PC106, n = 7; HyMad1, n = 7). Control group mice were treated with PBS using the same twice a day dose (PC106, n = 7; HyMad1 n = 7). T2 images of these mice were acquired weekly to quantify tumor size and evaluate changes in tumor growth over time. HP DHA imaging of pancreatic cancer PDXs – Ascorbate and vehicle-treated mice of both tumor models were injected with 250 μL of 30 mM HP DHA in D2O to investigate the redox status of the implanted tumor over time. 13C HP MRS was performed using an axial 2D CSI acquisition on a 5T small animal MRI spectrometer to measure DHA/ascorbate metabolism. DHA/ascorbate ratios were quantified by taking the area under the curve and compared between ascorbate-treated and vehicle mice to determine changes in tumor redox upon ascorbate therapy. Metabolic profiling of glucose consumption – Mouse embryonic fibroblasts (MEFs) with ATM and CHEK2 mutations were treated for 4 hours in RPMI media supplemented with 25 mM [1,2-13C2]-glucose, 100 μM sodium ascorbate and 10% dialyzed FBS (N=3). Cells treated with vehicle were supplemented with an equal volume of phosphate buffered saline instead of 100 uM sodium ascorbate (N=3). 500 μL of media was taken from each well and mixed with 100 μL D2O with 1 mM 13C urea used as reference for chemical shift and concentration determination. 13C experiments were acquired to determine the relative peak area under the curves (AUC) for C3-labeled lactate, C2,C3-doubly labelled lactate, and 13C urea. Absolute PPP activity and glycolytic activity was then calculated based on measured cell count for each well.Results and Discussion
High dose ascorbate therapy drives oxidate stress, leading to positive treatment response in a BRCA-mutant and KRAS-mutant model of cancer. Ascorbate treatment was shown to slow tumor growth in a KRAS-driven PDX model after 3 weeks while the effect on tumor growth was significant in a BRCA-driven PDX model after 4 days (Figure 1A). The observed ratios of HP ascorbate/total carbon match with the observed treatment response after 1 week in all 3 models (Figure 1B). Metabolomic studies using mass spectroscopy showed decreased levels of PPP intermediates in the rapidly responding BRCA PDX model, revealing that ascorbate therapy-mediated oxidative stress drives increased flux through the PPP (Figure 2). Metabolic profiling of glucose consumption shows increased PPP activity upon ascorbate treatment in wildtype and DNA damage repair mutant MEFs (Figure 3). Interestingly, ATM-mutant and CHK-mutant MEFs showed lower levels of glycolytic activity and baseline PPP activity than the parent wildtype MEFs. These results, in concert with the observed readouts of oxidative stress, demonstrate HP DHA as an effective biomarker for oxidative stress in tumors.Conclusion
We demonstrated HP DHA as an imaging agent for probing the biochemical mechanism of response to ascorbate therapy in PDX models of cancer. Changes in DHA/ascorbate metabolism were observed in these tumor models after prolonged treatment, mirroring the observed treatment response to high dose ascorbate. Metabolomic studies then showed that high dose ascorbate therapy induced oxidative stress, leading to increased flux through the PPP. DNA damage repair mutants exhibit lower baseline flux through the PPP and are more sensitized to ascorbate induced ROS. These results demonstrate a new method for assessing oxidative stress in vivo using HP DHA.Acknowledgements
The authors acknowledge the Stand Up to Cancer Foundation, Starr Cancer Consortium, Thompson Family Foundation, NIH/NCI Cancer Center Support Grant P30 CA008748 and NIH R01 CA 252037 for funding and support.References
1. Yun, J., Mullarky, E., Lu, C., Bosch, K. N., Kavalier, A., Rivera, K., Roper, J., Chio, I. I. C., Giannopoulou, E. G., Rago, C., Muley, A., Asara, J. M., Paik, J., Elemento, O., Chen, Z., Pappin, D. J., Dow, L. E., Papadopoulos, N., Gross, S. S., & Cantley, L. C. (2015). Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science, 350(6266), 1391-1396. doi:10.1126/science.aaa50042. Keshari, K. R., Sai, V., Wang, Z. J., Vanbrocklin, H. F., Kurhanewicz, J., & Wilson, D. M. (2013). Hyperpolarized [1-13C]dehydroascorbate MR spectroscopy in a murine model of prostate cancer: comparison with 18F-FDG PET. Journal of Nuclear Medicine, 54(6), 922–928. doi:10.2967/jnumed.112.115402
3. Keshari, K. R., Kurhanewicz, J., Bok, R., Larson, P. E., Vigneron, D. B., & Wilson, D. M. (2011). Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America, 108(46), 18606–18611. doi:10.1073/pnas.1106920108
4. Cheng, D. T., Mitchell, T. N., Zehir, A., Shah, R. H., Benayed, R., Syed, A., … Berger, M. F. (2015). Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of Molecular Diagnostics, 17(3), 251–264. doi:10.1016/j.jmoldx.2014.12.006
5. Mattar, M., McCarthy, C. R., Kulick, A. R., Qeriqi, B., Guzman, S., & de Stanchina, E. (2018). Establishing and Maintaining an Extensive Library of Patient-Derived Xenograft Models. Frontiers in Oncology, 8, 19. doi:10.3389/fonc.2018.00019
Figures
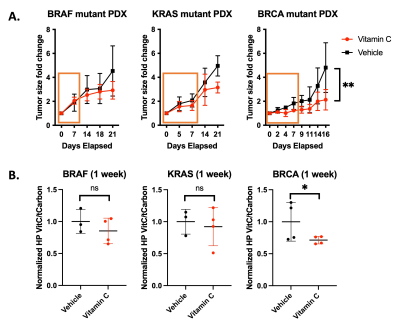
Treatment response to high dose
ascorbate in PDX models of cancer. (A) Tumor
growth change for the KRAS PDX
tumors is significant after 21 days and significant after 4 days for the BRCA
PDX tumors. (B)
Hyperpolarized MRS using [1-13C]dehydroascorbate
(DHA) reveals metabolic conversion
of DHA to ascorbate in pancreatic cancer PDXs mirrors
treatment response.
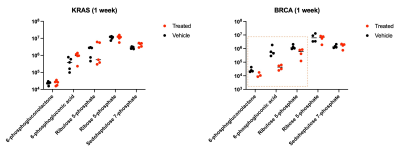
Metabolomics of KRAS and BRCA
driven PDX tumors treated with high dose ascorbate and phosphate buffered
saline vehicle. A BRCA PDX model that responds to
ascorbate treatment exhibits decreased levels of PPP intermediates, revealing
that ascorbate-mediated oxidative stress drives increased flux through the
pentose phosphate pathway.
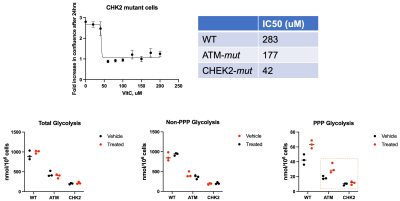
MEFs lacking DNA damage response
enzymes are more sensitive to ascorbate treatment. Metabolic
profiling of glucose consumption shows increased PPP activity upon ascorbate
treatment in wildtype and DNA damage repair mutant MEFs.
DOI: https://doi.org/10.58530/2022/0831